Shear-Wave Elastography Variability Analysis and Relation with Kidney Allograft Dysfunction: A Single-Center Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Design of Experiment
2.2. Clinical Evaluation
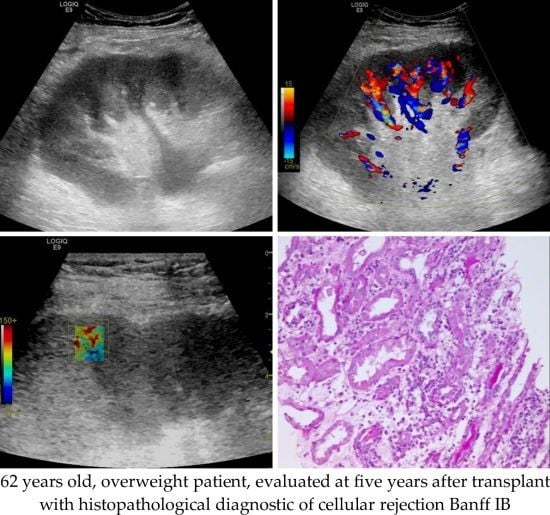
2.3. Grey Scale and Doppler Examination
2.4. Shear Wave Elastography Examination
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- United Network for Organ Sharing—Transplant Trends. Available online: https://unos.org/data/transplant-trends/ (accessed on 27 September 2019).
- Vanholder, R.; Stel, V.S.; Jager, K.J.; Lameire, N.; Loud, F.; Oberbauer, R.; de Jong, R.W.; Zoccali, C. How to increase kidney transplant activity throughout Europe—An advocacy review by the European Kidney Health Alliance. Nephrol. Dial. Transplant. 2019, 34, 1254–1261. [Google Scholar] [CrossRef]
- Deurdulian, C.; Tchelepi, H. Chapter 27. Imaging-based monitoring of the renal graft. In Kidney Transplantation, Bioengineering and Regeneration; Orland, G., Remuzzi, G., Williams, D.F., Eds.; Academic Press, Elsevier: London, UK, 2017; pp. 373–402. [Google Scholar]
- Langer, J.; Jones, L. Sonographic evaluation of the renal transplant. Ultrasound Clin. 2007, 2, 73–83. [Google Scholar] [CrossRef]
- Gabriel, H.; Sun, M.R.; De Jesus Allison, S.O.; Sheth, S.; Teefey, S.A.; Wagner, J.M.; Bluth, E.; Grant, E.G.; Scoutt, L.; Hamper, U.M.; et al. AIUM practice guideline for the performance of an ultrasound examination of solid-organ transplants. J. Ultrasound Med. 2014, 33, 1309–1320. [Google Scholar] [CrossRef] [Green Version]
- Patel, U.; Khaw, K.K.; Hughes, N.C. Doppler ultrasound for detection of renal transplant artery stenosis: Threshold peak systolic velocity needs to be higher in a low-risk or surveillance population. Clin. Radiol. 2003, 58, 772–777. [Google Scholar] [CrossRef]
- Köhnke, R.; Kentrup, D.; Schütte-Nütgen, K.; Schäfers, M.; Schnöckel, U.; Hoerr, V.; Reuter, S. Update on imaging-based diagnosis of acute renal allograft rejection. Am. J. Nucl. Med. Mol. Imaging 2019, 9, 110–126. [Google Scholar]
- Álvarez Rodríguez, S.; Hevia Palacios, V.; Sanz Mayayo, E.; Gómez Dos Santos, V.; Díez Nicolás, V.; Sánchez Gallego, M.D.; Lorca Álvaro, J.; Burgos Revilla, F.J. The usefulness of contrast-enhanced ultrasound in the assessment of early kidney transplant function and complications. Diagnostics 2017, 7, 53. [Google Scholar] [CrossRef]
- Tatar, I.G.; Teber, M.A.; Ogur, T.; Kurt, A.; Hekimoglu, B. Real time sonoelastographic evaluation of renal allografts in correlation with clinical prognostic parameters: Comparison of linear and convex transducers according to segmental anatomy. Med. Ultrason. 2014, 16, 229–235. [Google Scholar] [CrossRef] [Green Version]
- Samir, A.E.; Allegretti, A.S.; Zhu, Q.; Dhyani, M.; Anvari, A.; Sullivan, D.A.; Trottier, C.A.; Dougherty, S.; Williams, W.W.; Babitt, J.L.; et al. Shear wave elastography in chronic kidney disease: A pilot experience in native kidneys. BMC Nephrol. 2015, 16, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, K.; Loberant, N.; Abbas, N.; Fadi, H.; Shadia, H.; Khazim, K. Shear wave elastography imaging for assessing the chronic pathologic changes in advanced diabetic kidney disease. Ther. Clin. Risk Manag. 2016, 12, 1615–1622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bob, F.; Bota, S.; Sporea, I.; Sirli, R.; Popescu, A.; Schiller, A. Relationship between the estimated glomerular filtration rate and kidney shear wave speed values assessed by acoustic radiation force impulse elastography: A pilot study. J. Ultrasound Med. 2015, 34, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Leong, S.S.; Wong, J.H.D.; Md Shah, M.N.; Vijayananthan, A.; Jalalonmuhali, M.; Ng, K.H. Shear wave elastography in the evaluation of renal parenchymal stiffness in patients with chronic kidney disease. Br. J. Radiol. 2018, 91, 20180235. [Google Scholar] [CrossRef] [PubMed]
- Syversveen, T.; Brabrand, K.; Midtvedt, K.; Strøm, E.H.; Hartmann, A.; Jakobsen, J.A.; Berstad, A.E. Assessment of renal allograft fibrosis by acoustic radiation force impulse quantification: A pilot study. Transpl. Int. 2011, 24, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Syversveen, T.; Midtvedt, K.; Berstad, A.E.; Brabrand, K.; Strom, E.H.; Abildgaard, A. Tissue elasticity estimated by acoustic radiation force impulse quantification depends on the applied transducer force: An experimental study in kidney transplant patients. Eur. Radiol. 2012, 22, 2130–2137. [Google Scholar] [CrossRef] [PubMed]
- Stock, K.F.; Klein, B.S.; Vo Cong, M.T.; Sarkar, O.; Römisch, M.; Regenbogen, C.; Büttner, M.; Schuster, T.; Matevossian, E.; Amann, K.; et al. ARFI-based tissue elasticity quantification in comparison to histology for the diagnosis of renal transplant fibrosis. Clin. Hemorheol. Microcirc. 2010, 46, 139–148. [Google Scholar] [CrossRef] [PubMed]
- He, W.Y.; Jin, Y.J.; Wang, W.P.; Li, C.L.; Ji, Z.B.; Yang, C. Tissue elasticity quantification by acoustic radiation force impulse for the assessment of renal allograft function. Ultrasound Med. Biol. 2014, 40, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.Z.; Li, C.L.; Xu, H.; Zhang, Y.; Niu, H.; Li, X.M. The primary application of acoustic radiation force impulse in transplanted kidneys. Chin. J. Med. Ultrasound (Electronic Ed) 2013, 10, 227–230. [Google Scholar]
- Early, H.M.; Cheang, E.C.; Aguilera, J.M.; Hirschbein, J.S.W.; Fananapazir, G.; Wilson, M.D.; McGahan, J.P. Utility of shear wave elastography for assessing allograft fibrosis in renal transplant recipients: A pilot study. J. Ultrasound Med. 2018, 37, 1455–1465. [Google Scholar] [CrossRef]
- Lee, J.; Oh, Y.T.; Joo, D.J.; Ma, B.G.; Lee, A.L.; Lee, J.G.; Song, S.H.; Kim, S.U.; Jung, D.C.; Chung, Y.E.; et al. Acoustic radiation force impulse measurement in renal transplantation: A prospective, longitudinal study with protocol biopsies. Medicine (Baltimore) 2015, 94, e1590. [Google Scholar] [CrossRef]
- Early, H.; Aguilera, J.; Cheang, E.; McGahan, J. Challenges and considerations when using shear wave elastography to evaluate the transplanted kidney, with pictorial review. J. Ultrasound Med. 2017, 36, 1771–1782. [Google Scholar] [CrossRef] [Green Version]
- Ghonge, N.P.; Mohan, M.; Kashyap, V.; Jasuja, S. Renal allograft dysfunction: Evaluation with shear-wave sonoelastography. Radiology 2018, 288, 146–152. [Google Scholar] [CrossRef]
- Ma, M.K.; Law, H.K.; Tse, K.S.; Chan, K.W.; Chan, G.C.; Yap, D.Y.; Mok, M.M.; Kwan, L.P.; Tang, S.C.; Choy, B.Y.; et al. Non-invasive assessment of kidney allograft fibrosis with shear wave elastography: A radiological-pathological correlation analysis. Int. J. Urol. 2018, 25, 450–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Yang, H.; Suo, C.; Wei, J.; Tan, R.; Gu, M. Application of ultrasound elastography for chronic allograft dysfunction in kidney transplantation. J. Ultrasound Med. 2017, 36, 1759–1769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bromberg, J.S.; Fairchild, R.L.; Feng, S.; Kaplan, B.; Barr, M.L.; Grady, J.O. Kidney disease: Improving global outcomes (KDIGO) transplant work group. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am. J. Transplant. 2009, 9, S1–S157. [Google Scholar]
- Evaluation and Diagnosis of the Patient with Renal Allograft Dysfunction—UpToDate. Available online: https://www.uptodate.com/contents/evaluation-and-diagnosis-of-the-patient-with-renal-allograft-dysfunction?search=kidneytransplant&source=search_result&selectedTitle=4~150&usage_type=default&display_rank=4 (accessed on 11 August 2019).
- Eknoyan, G. Adolphe quetelet (1796–1874)-the average man and indices of obesity. Nephrol. Dial. Transplant. 2008, 23, 47–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.; Castro, A.F.; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Int. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Loupy, A.; Haas, M.; Solez, K.; Racusen, L.; Glotz, D.; Seron, D.; Nankivell, B.J.; Colvin, R.B.; Afrouzian, M.; Akalin, E.; et al. The banff 2015 kidney meeting report: Current challenges in rejection classification and prospects for adopting molecular pathology. Am. J. Transplant. 2017, 17, 28–41. [Google Scholar] [CrossRef]
- Zakhari, N.; Blew, B.; Shabana, W. Simplified method to measure renal volume: The best correction factor for the ellipsoid formula volume calculation in pretransplant computed tomographic live donor. Urology 2014, 83, 1444.e15–1444.e19. [Google Scholar] [CrossRef]
- Nightingale, K.; McAleavey, S.; Trahey, G. Shearwave generation using acoustic radiation force: In vivo and ex vivo results. Ultrasound Med. Biol. 2003, 29, 1715–1723. [Google Scholar] [CrossRef]
- Bolboacă, S.D. Medical diagnostic tests: A review of test anatomy, phases, and statistical treatment of data. Comput. Math. Methods Med. 2019, 2019, 1891569. [Google Scholar] [CrossRef] [Green Version]
- Altunkaynak, B.; Gamgam, H. Bootstrap confidence intervals for the coefficient of quartile variation. Commun. Stat. Simul. Comput. 2018, 48, 2138–2146. [Google Scholar] [CrossRef]
- Nankivell, B.J.; Borrows, R.J.; Fung, C.L.; O’Connell, P.J.; Allen, R.D.; Chapman, J.R. The natural history of chronic allograft nephropathy. N. Engl. J. Med. 2003, 349, 2326–2333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breda, A.; Budde, K.; Figueiredo, A.; Lledó García, E.; Olsburgh, J.; Regele, H. EAU Guidelines on Renal Transplantation. 2017. Available online: https://uroweb.org/wp-content/uploads/Renal-Transplantation-2017-pocket.pdf (accessed on 10 November 2019).
- Karam, G.; Kälble, T.; Alcaraz, A.; Aki, F.T.; Budde, K.; Humke, U.; Kleinclauss, F.; Nicita, G.; Olsburgh, J.O.; Süsal, C. Guidelines on Renal Transplantation. European Association of Urology 2014. Available online: https://uroweb.org/wp-content/uploads/EAU-Guidelines-Renal-Transplantation-2009.pdf (accessed on 10 November 2019).
- Järv, L.; Kull, I.; Riispere, Z.; Kuudeberg, A.; Lember, M.; Ots-Rosenberg, M. Ultrasound elastography correlations between anthropometrical parameters in kidney transplant recipients. J. Investig. Med. 2019, 67, 1137–1141. [Google Scholar] [CrossRef] [PubMed]
- Boas, F.E.; Desser, T.S.; Kamaya, A. Does separating the resistive index into pre- and post-glomerular resistance and vascular compliance improve the diagnostic accuracy of renal transplant Doppler ultrasound? Am. J. Roentgenol. 2011, 196, A87. [Google Scholar]
- Meier, M.; Fricke, L.; Eikenbusch, K.; Smith, E.; Kramer, J.; Lehnert, H.; Nitschke, M. The serial duplex index improves differential diagnosis of acute renal transplant dysfunction. J. Ultrasound Med. 2017, 36, 1607–1615. [Google Scholar] [CrossRef] [PubMed]
- Naesens, M.; Heylen, L.; Lerut, E.; Claes, K.; De Wever, L.; Claus, F.; Oyen, R.; Kuypers, D.; Evenepoel, P.; Bammens, B.; et al. Intrarenal resistive index after renal transplantation. N. Engl. J. Med. 2013, 369, 1797–1806. [Google Scholar] [CrossRef] [PubMed]
- Viazzi, F.; Leoncini, G.; Derchi, L.E.; Pontremoli, R. Ultrasound doppler renal resistive index: A useful tool for the management of the hypertensive patient. J. Hypertens. 2014, 32, 149–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radermacher, J.; Mengel, M.; Ellis, S.; Stuht, S.; Hiss, M.; Schwarz, A.; Eisenberger, U.; Burg, M.; Luft, F.C.; Gwinner, W.; et al. The renal arterial resistance index and renal allograft survival. N. Engl. J. Med. 2003, 349, 115–124. [Google Scholar] [CrossRef] [Green Version]
- Kramann, R.; Frank, D.; Brandenburg, V.M.; Heussen, N.; Takahama, J.; Krüger, T.; Riehl, J.; Floege, J. Prognostic impact of renal arterial resistance index upon renal allograft survival: The time point matters. Nephrol. Dial. Transplant. 2012, 27, 3958–3963. [Google Scholar] [CrossRef] [Green Version]
- El-Motaal, A.M.A.; Dawoud, R.M.; Sherif, M.F.; Eldiasty, T.A. Role of ultrasound, color duplex doppler and sono-elastography in the evaluation of renal allograft complications. Egypt. J. Radiol. Nucl. Med. 2019, 50, 83. [Google Scholar] [CrossRef]
- Zwillinger, D.; Kokoska, S. Standard Probability and Statistical Tables and Formula; Chapman & Hall: Boca Raton, FL, USA, 2000. [Google Scholar]
- Wild, D. The Immunoassay Handbook: Theory and Applications of Ligand Binding, ELISA and Related Techniques, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Steel, R.G.D.; Torrie, J.H. Principles and Procedures of Statistics, 2nd ed.; McGraw-Hill: New York, NY, USA, 1980. [Google Scholar]
- Grenier, N.; Poulain, S.; Lepreux, S.; Gennisson, J.L.; Dallaudière, B.; Lebras, Y.; Bavu, E.; Servais, A.; Meas-Yedid, V.; Piccoli, M.; et al. Quantitative elastography of renal transplants using supersonic shear imaging: A pilot study. Eur. Radiol. 2012, 22, 2138–2146. [Google Scholar] [CrossRef]
- Bercoff, J.; Tanter, M.; Fink, M. Supersonic shear imaging: A new technique for soft tissue elasticity mapping. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2004, 51, 396–409. [Google Scholar] [CrossRef] [PubMed]
- Arndt, R.; Schmidt, S.; Loddenkemper, C.; Grünbaum, M.; Zidek, W.; van der Giet, M.; Westhoff, T.H. Noninvasive evaluation of renal allograft fibrosis by transient elastography: A pilot study. Transpl. Int. 2010, 23, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, F.; Pan, W.; Ruan, L.; Ai, H. Correlation between ultrasound elastography parameters and renal function after kidney transplantation. Int. J. Clin. Exp. Med. 2017, 10, 3211–3217. [Google Scholar]
- Brocchi, S.; Sportoletti, C.; Serra, C.; Zompatori, M. Shearwave Elastography in Kidney Transplantation: A New Diagnostic Tool to Assess Chronic Allograft Fibrosis. ECR 2014/C-1199. Available online: https://posterng.netkey.at/esr/viewing/index.php?module=viewing_poster&task=&pi=120983 (accessed on 10 November 2019).





| Parameter | Kidney Recipient | Donor |
|---|---|---|
| Age at kidney transplant, years | 48 (37–55) {23 to 64} | 55 (47–62.5) {13 to 72} |
| Women, no. (%) | 22 (37.3) | 27 (45.8) |
| BMI, kg/m2 | 25 (22.5–28.5) {18 to 33} | |
| BMI classification, no. (%) | ||
| Normal weight | 29 (49.2) | |
| Overweight | 17 (28.8) | |
| Obesity | 13 (22.0) | |
| Mismatch HLA | 5 (4–5) {2 to 6} | |
| Ac-anti-HLA class I, no. (%) | 5 (8.5) | |
| Ac-anti-HLA class II, no. (%) | 6 (10.2) | |
| Alive, no. (%) | 10 (16.9) | 10 (16.9) |
| Cause of death, no. (%) | ||
| Stroke | 32 (54.2) | 32 (54.2) |
| Craniocerebral trauma | 11 (18.6) | 11 (18.6) |
| Suicide | 4 (6.8) | 4 (6.8) |
| Cardiorespiratory arrest | 2 (3.4) | 2 (3.4) |
| Parameter | All, n = 83 | Dysfunction, n = 14 | Stable Function, n = 69 | Statistics (p-Value) |
|---|---|---|---|---|
| Serum creatinine level, mg/dL | 1.46 (1.19–1.96) | 3.71 (2.66–5.10) | 1.33 (1.16–1.63) | 5.09 (<0.0001) |
| eGRF (mL/mim/1.73 m2) | 52 (32–64) | 16 (12–30) | 54 (43–69) | −4.95 (<0.0001) |
| Proteinuria/Creatinuria ratio | 0.200 (0.106–0.997) | 2.145 (1.483–7.603) | 0.160 (0.095–0.380) | 4.45 (<0.0001) |
| Calcineurin inhibitor | n.a. (0.0037) | |||
| Normal | 49 (61.3) | 3 (25.0) | 46 (67.6) | |
| Suboptimal dose | 15 (18.8) | 6 (50.0) | 9 (13.2) | |
| Over dose | 16 (20.0) | 3 (25.0) | 13 (19.1) |
| Parameter | All, n = 83 | Dysfunction, n = 14 | Stable Function, n = 69 | Stat. (p-Value) |
|---|---|---|---|---|
| Allograft volume, ml | 186 (150–226.5) | 186 (164.8–247.5) | 186 (150–225) | 0.27 (0.7844) |
| Parenchymal thickness (mm) | 14.7 (13.2–16.7) | 14.1 (13.2–14.6) | 15.0 (13.2–16.7) | −1.01 (0.3128) |
| Cortical thickness (mm) | 7.5 (6.6–8.6) | 6.9 (5.6–8.4) | 7.7 (6.8–8.6) | −1.26 (0.2060) |
| Echogenicity | n.a. (0.0113) | |||
| Normal | 65 (78.3) | 7 (50.0) | 58 (84.1) | |
| High cortical-medullar diff | 14 (16.9) | 5 (35.7) | 9 (13.0) | |
| Diffuse hyperechogenicity | 4 (4.8) | 2 (14.3) | 2 (2.9) | |
| Resistivity Index | 0.63 | 0.60 | 0.64 | −0.86 (0.3913) |
| Upper interlobar artery | (0.58–0.69) | (0.58–0.66) | (0.58–0.69) | |
| Resistivity Index | 0.66 | 0.64 | 0.66 | −0.51 (0.6095) |
| Medium interlobar artery | (0.62–0.70) | (0.59–0.74) | (0.63–0.69) | |
| Resistivity Index | 0.66 | 0.64 | 0.66 | −0.52 (0.6010) |
| Lower interlobar artery | (0.62–0.71) | (0.62–0.70) | (0.62–0.71) |
| All, n = 83 | Dysfunction, n = 14 | Stable Function, n = 59 | ||||
|---|---|---|---|---|---|---|
| Cortex | Medulla | Cortex | Medulla | Cortex | Medulla | |
| A1 | 34.43 (27.42–41.76) | 33.08 (20.19–47.96) | 38.19(23.24–64.92) | 43.55(24.35–74.75) | 30.99 (23.63–39.32) | 33.96 (17.93–51.91) |
| A2 | 30.99 (23.72–43.10) | 42.38 (33.00–53.18) | 34.15 (16.94–53.57) | 39.19 (17.51–66.43) | 28.70 (19.49–41.85) | 40.90 (29.01–52.32) |
| A3 | 31.98 (24.39–40.99) | 33.16 (21.08–45.90) | 26.17 (13.01–45.18) | 28.91 (7.65–66.16) | 31.61 (22.82–40.57) | 33.90 (21.24–45.45) |
| A4 | 28.66 (23.77–36.15) | 40.20 (28.10–49.22) | 19.33 (4.50–46.02) | 36.59 (11.25–64.63) | 28.83 (22.69–38.20) | 39.06 (27.74–49.65) |
| A5 | 30.37 (22.02–40.49) | 34.32 (23.41–45.70) | 23.99 (9.84–54.64) | 37.72 (20.86–81.13) | 29.29 (22.20–40.93) | 32.22 (20.25–45.05) |
| B1 | 29.78 (24.53–37.89) | 37.34 (30.03–45.86) | 22.91 (7.40–41.12) | 30.95 (11.02–51.05) | 27.91 (17.00–35.27) | 38.83 (30.89–48.38) |
| B2 | 32.00 (26.18–38.84) | 36.04 (27.19–43.38) | 17.53 (7.64–37.44) | 27.09 (15.28–49.70) | 29.76 (21.90–35.88) | 38.92 (29.92–48.86) |
| B3 | 28.91 (24.23–34.36) | 40.87 (32.85–50.50) | 21.91 (3.85–39.25) | 31.63 (12.99–57.28) | 28.09 (22.28–34.44) | 40.57 (30.72–52.00) |
| B4 | 28.79 (23.12–34.20) | 31.54 (21.98–40.83) | 24.64 (12.52–54.28) | 24.81 (8.86–41.99) | 25.87 (19.21–32.26) | 32.11 (22.08–44.20) |
| B5 | 33.9 (26.22–42.93) | 39.12 (30.61–48.38) | 21.19 (11.69–46.57) | 17.59 (1.59–36.54) | 32.77 (23.32–41.77) | 38.79 (27.13–48.24) |
| Median | 27.34 (21.52–33.01) | 30.81 (23.29–41.43) | 24.64 (13.20–54.65) | 24.81 (11.50–49.53) | 26.67 (21.71–35.13) | 28.56 (18.07–39.55) |
| Parameter | All Cohort, n = 83 | Dysfunction, n = 11 | Stable Function, n = 72 |
|---|---|---|---|
| BMI & YM cortex | −0.44 (<0.0001) | −0.47 (0.1412) | −0.39 (0.0008) |
| BMI & YM medulla | −0.42 (0.0001) | −0.45 (0.1691) | −0.38 (0.0010) |
| TimeTE & YM cortex | 0.36 (0.0008) | 0.45 (0.1601) | 0.30 (0.0110) |
| PCR & YM cortex | 0.33 (0.0021) | 0.05 (0.8734) | 0.27 (0.0200) |
| PCR & YM medulla | 0.28 (0.0105) | 0.18 (0.5926) | 0.24 (0.0436) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolboacă, S.D.; Elec, F.I.; Elec, A.D.; Muntean, A.M.; Socaciu, M.A.; Iacob, G.; Zaro, R.; Andrieș, A.-I.; Bădulescu, R.M.; Ignat, R.M.; et al. Shear-Wave Elastography Variability Analysis and Relation with Kidney Allograft Dysfunction: A Single-Center Study. Diagnostics 2020, 10, 41. https://doi.org/10.3390/diagnostics10010041
Bolboacă SD, Elec FI, Elec AD, Muntean AM, Socaciu MA, Iacob G, Zaro R, Andrieș A-I, Bădulescu RM, Ignat RM, et al. Shear-Wave Elastography Variability Analysis and Relation with Kidney Allograft Dysfunction: A Single-Center Study. Diagnostics. 2020; 10(1):41. https://doi.org/10.3390/diagnostics10010041
Chicago/Turabian StyleBolboacă, Sorana D., Florin Ioan Elec, Alina Daciana Elec, Adriana Milena Muntean, Mihai Adrian Socaciu, Gheorghita Iacob, Răzvan Zaro, Alexandra-Ioana Andrieș, Ramona Maria Bădulescu, Radu Mihai Ignat, and et al. 2020. "Shear-Wave Elastography Variability Analysis and Relation with Kidney Allograft Dysfunction: A Single-Center Study" Diagnostics 10, no. 1: 41. https://doi.org/10.3390/diagnostics10010041