Enhancing Conversion Efficiency of Direct Ink Write Printed Copper (I) Sulfide Thermoelectrics via Sulfur Infusion Process
Abstract
:1. Introduction
2. Materials and Methods
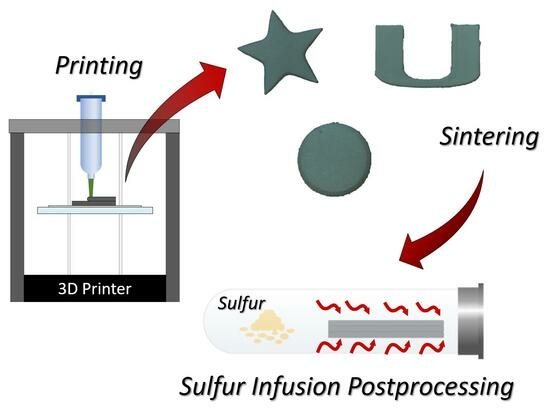
2.1. Ink Preparation and 3D Printing
2.2. Sintering and Sulfur Diffusion Post-Processing
2.3. Characterization of Crystal Structure and Sulfur Content
2.4. Thermoelectric Characterization
3. Results and Discussion
3.1. Structural and Morphological Analysis
3.2. Compositional Analysis
3.3. Thermoelectric Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Twaha, S.; Zhu, J.; Yan, Y.; Li, B. A comprehensive review of thermoelectric technology: Materials, applications, modelling and performance improvement. Renew. Sustain. Energy Rev. 2016, 65, 698–726. [Google Scholar] [CrossRef]
- Pourkiaei, S.M.; Ahmadi, M.H.; Sadeghzadeh, M.; Moosavi, S.; Pourfayaz, F.; Chen, L.; Pour Yazdi, M.A.; Kumar, R. Thermoelectric cooler and thermoelectric generator devices: A review of present and potential applications, modeling and materials. Energy 2019, 186, 115849. [Google Scholar] [CrossRef]
- Goldsmid, H.J. Bismuth Telluride and Its Alloys as Materials for Thermoelectric Generation. Materials 2014, 7, 2577–2592. [Google Scholar] [CrossRef]
- Poudel, B.; Hao, Q.; Ma, Y.; Lan, Y.C.; Minnich, A.; Yu, B.; Yan, X.A.; Wang, D.Z.; Muto, A.; Vashaee, D.; et al. High-thermoelectric performance of nanostructured bismuth antimony telluride bulk alloys. Science 2008, 320, 634–638. [Google Scholar] [CrossRef]
- Witting, I.T.; Chasapis, T.C.; Ricci, F.; Peters, M.; Heinz, N.A.; Hautier, G.; Snyder, G.J. The Thermoelectric Properties of Bismuth Telluride. Adv. Electron. Mater. 2019, 5, 1800904. [Google Scholar] [CrossRef]
- Yamashita, O.; Tomiyoshi, S.; Makita, K. Bismuth telluride compounds with high thermoelectric figures of merit. J. Appl. Phys. 2003, 93, 368–374. [Google Scholar] [CrossRef]
- Zhang, H.; Hobbis, D.; Nolas, G.S.; LeBlanc, S. Laser additive manufacturing of powdered bismuth telluride. J. Mater. Res. 2018, 33, 4031–4039. [Google Scholar] [CrossRef]
- Dughaish, Z.H. Lead telluride as a thermoelectric material for thermoelectric power generation. Phys. B 2002, 322, 205–223. [Google Scholar] [CrossRef]
- LaLonde, A.D.; Pei, Y.Z.; Wang, H.; Snyder, G.J. Lead telluride alloy thermoelectrics. Mater. Today 2011, 14, 526–532. [Google Scholar] [CrossRef]
- Pei, Y.Z.; Heinz, N.A.; LaLonde, A.; Snyder, G.J. Combination of large nanostructures and complex band structure for high performance thermoelectric lead telluride. Energy Environ. Sci. 2011, 4, 3640–3645. [Google Scholar] [CrossRef]
- Liu, H.; Shi, X.; Xu, F.; Zhang, L.; Zhang, W.; Chen, L.; Li, Q.; Uher, C.; Day, T.; Snyder, G.J. Copper ion liquid-like thermoelectrics. Nat. Mater. 2012, 11, 422–425. [Google Scholar] [CrossRef] [PubMed]
- Mulla, R.; Rabinal, M.H.K. Copper Sulfides: Earth-Abundant and Low-Cost Thermoelectric Materials. Energy Technol. 2019, 7, 1800850. [Google Scholar] [CrossRef]
- Oztan, C.Y.; Hamawandi, B.; Zhou, Y.; Ballikaya, S.; Toprak, M.S.; Leblanc, R.M.; Coverstone, V.; Celik, E. Thermoelectric performance of Cu2Se doped with rapidly synthesized gel-like carbon dots. J. Alloys Compd. 2021, 864, 157916. [Google Scholar] [CrossRef]
- Kang, S.D.; Pöhls, J.-H.; Aydemir, U.; Qiu, P.; Stoumpos, C.C.; Hanus, R.; White, M.A.; Shi, X.; Chen, L.; Kanatzidis, M.G.; et al. Enhanced stability and thermoelectric figure-of-merit in copper selenide by lithium doping. Mater. Today Phys. 2017, 1, 7–13. [Google Scholar] [CrossRef]
- Shi, D.-l.; Geng, Z.-m.; Shi, L.; Li, Y.; Lam, K.-h. Thermal stability study of Cu1.97Se superionic thermoelectric materials. J. Mater. Chem. C 2020, 8, 10221–10228. [Google Scholar] [CrossRef]
- Liu, W.D.; Yang, L.; Chen, Z.G.; Zou, J. Promising and Eco-Friendly Cu2X-Based Thermoelectric Materials: Progress and Applications. Adv. Mater. 2020, 32, e1905703. [Google Scholar] [CrossRef]
- Okamoto, K.; Kawai, S. Electrical Conduction and Phase Transition of Copper Sulfides. Jpn. J. Appl. Phys. 1973, 12, 1130. [Google Scholar] [CrossRef]
- Nieroda, P.; Leszczyński, J.; Mikuła, A.; Mars, K.; Kruszewski, M.J.; Koleżyński, A. Thermoelectric properties of Cu2S obtained by high temperature synthesis and sintered by IHP method. Ceram. Int. 2020, 46, 25460–25466. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, X.; Fei, F.Y.; Wang, J.; Cheng, Z.; Dou, S.; Wang, J.; Snyder, G.J. High thermoelectric and mechanical performance in highly dense Cu2−xS bulks prepared by a melt-solidification technique. J. Mater. Chem. A 2015, 3, 9432–9437. [Google Scholar] [CrossRef]
- Chakrabarti, D.J.; Laughlin, D.E. The Cu-S (Copper-Sulfur) system. Bull. Alloy Phase Diagr. 1983, 4, 254–271. [Google Scholar] [CrossRef]
- He, Y.; Day, T.; Zhang, T.; Liu, H.; Shi, X.; Chen, L.; Snyder, G.J. High thermoelectric performance in non-toxic earth-abundant copper sulfide. Adv. Mater. 2014, 26, 3974–3978. [Google Scholar] [CrossRef] [PubMed]
- Dennler, G.; Chmielowski, R.; Jacob, S.; Capet, F.; Roussel, P.; Zastrow, S.; Nielsch, K.; Opahle, I.; Madsen, G.K.H. Are Binary Copper Sulfides/Selenides Really New and Promising Thermoelectric Materials? Adv. Energy Mater. 2014, 4, 1301581. [Google Scholar] [CrossRef]
- Wei, T.-R.; Qin, Y.; Deng, T.; Song, Q.; Jiang, B.; Liu, R.; Qiu, P.; Shi, X.; Chen, L. Copper chalcogenide thermoelectric materials. Sci. China Mater. 2018, 62, 8–24. [Google Scholar] [CrossRef]
- Li, M.; Liu, Y.; Zhang, Y.; Han, X.; Zhang, T.; Zuo, Y.; Xie, C.; Xiao, K.; Arbiol, J.; Llorca, J.; et al. Effect of the Annealing Atmosphere on Crystal Phase and Thermoelectric Properties of Copper Sulfide. ACS Nano 2021, 15, 4967–4978. [Google Scholar] [CrossRef] [PubMed]
- Qiu, P.; Agne, M.T.; Liu, Y.; Zhu, Y.; Chen, H.; Mao, T.; Yang, J.; Zhang, W.; Haile, S.M.; Zeier, W.G.; et al. Suppression of atom motion and metal deposition in mixed ionic electronic conductors. Nat. Commun. 2018, 9, 2910. [Google Scholar] [CrossRef]
- Oztan, C.; Ballikaya, S.; Ozgun, U.; Karkkainen, R.; Celik, E. Additive manufacturing of thermoelectric materials via fused filament fabrication. Appl. Mater. Today 2019, 15, 77–82. [Google Scholar] [CrossRef]
- Burton, M.; Howells, G.; Atoyo, J.; Carnie, M. Printed Thermoelectrics. Adv. Mater. 2022, 34, e2108183. [Google Scholar] [CrossRef]
- Kim, F.; Yang, S.E.; Ju, H.; Choo, S.; Lee, J.; Kim, G.; Jung, S.H.; Kim, S.; Cha, C.; Kim, K.T.; et al. Direct ink writing of three-dimensional thermoelectric microarchitectures. Nat. Electron. 2021, 4, 579–587. [Google Scholar] [CrossRef]
- Jo, S.; Choo, S.; Kim, F.; Heo, S.H.; Son, J.S. Ink Processing for Thermoelectric Materials and Power-Generating Devices. Adv. Mater. 2019, 31, 1804930. [Google Scholar] [CrossRef]
- Kato, K.; Hagino, H.; Miyazaki, K. Fabrication of Bismuth Telluride Thermoelectric Films Containing Conductive Polymers Using a Printing Method. J. Electron. Mater. 2013, 42, 1313–1318. [Google Scholar] [CrossRef]
- Amin, A.; Huang, R.; Newbrook, D.; Sethi, V.; Yong, S.; Beeby, S.; Nandhakumar, I. Screen-printed bismuth telluride nanostructured composites for flexible thermoelectric applications. J. Phys.-Energy 2022, 4, 024003. [Google Scholar] [CrossRef]
- Chen, B.L.; Kruse, M.; Xu, B.; Tutika, R.; Zheng, W.; Bartlett, M.D.; Wu, Y.; Claussen, J.C. Flexible thermoelectric generators with inkjet-printed bismuth telluride nanowires and liquid metal contacts. Nanoscale 2019, 11, 5222–5230. [Google Scholar] [CrossRef]
- Kim, F.; Kwon, B.; Eom, Y.; Lee, J.E.; Park, S.; Jo, S.; Park, S.H.; Kim, B.S.; Im, H.J.; Lee, M.H.; et al. 3D printing of shape-conformable thermoelectric materials using all-inorganic Bi2Te3-based inks. Nat. Energy 2018, 3, 301–309. [Google Scholar] [CrossRef]
- Burton, M.R.; Mehraban, S.; McGettrick, J.; Watson, T.; Lavery, N.P.; Carnie, M.J. Earth abundant, non-toxic, 3D printed Cu2−xS with high thermoelectric figure of merit. J. Mater. Chem. A 2019, 7, 25586–25592. [Google Scholar] [CrossRef]
- Pan, S.-H.; Yao, G.-C.; Cui, Y.-N.; Meng, F.-S.; Luo, C.; Zheng, T.-Q.; Singh, G. Additive manufacturing of tungsten, tungsten-based alloys, and tungsten matrix composites. Tungsten 2022, 5, 1–31. [Google Scholar] [CrossRef]
- Caccia, M.; Tabandeh-Khorshid, M.; Itskos, G.; Strayer, A.R.; Caldwell, A.S.; Pidaparti, S.; Singnisai, S.; Rohskopf, A.D.; Schroeder, A.M.; Jarrahbashi, D.; et al. Ceramic–metal composites for heat exchangers in concentrated solar power plants. Nature 2018, 562, 406–409. [Google Scholar] [CrossRef]
- Zohuriaan, M.J.; Shokrolahi, F. Thermal studies on natural and modified gums. Polym. Test. 2004, 23, 575–579. [Google Scholar] [CrossRef]
- Ramdohr, P. The Ore Minerals and Their Intergrowths; Elsevier Science: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Zheng, Y.; Xie, H.; Shu, S.; Yan, Y.; Li, H.; Tang, X. High-Temperature Mechanical and Thermoelectric Properties of p-Type Bi0.5Sb1.5Te3 Commercial Zone Melting Ingots. J. Electron. Mater. 2013, 43, 2017–2022. [Google Scholar] [CrossRef]
- Cook, W.R. Phase Changes in Cu2S as a Function of Temperature; National Bureau of Standards: Gaithersburg, MD, USA, 1972. [Google Scholar]
- Sorokin, G.P.; Paradenko, A.P. Electrical Properties of Cu2S. Sov. Phys. J. 1966, 9, 59–61. [Google Scholar] [CrossRef]
- Snyder, G.J.; Toberer, E.S. Complex thermoelectric materials. Nat. Mater. 2008, 7, 105–114. [Google Scholar] [CrossRef]
- Nieroda, P.; Ziewiec, K.; Leszczyński, J.; Rutkowski, P.; Koleżyński, A. Extremely Fast and Cheap Densification of Cu2S by Induction Melting Method. Materials 2021, 14, 7311. [Google Scholar] [CrossRef] [PubMed]
- Roseboom, E.H., Jr. An investigation of the system Cu-S and some natural copper sulfides between 25 and 700 C. Econ. Geol. 1966, 61, 641–672. [Google Scholar] [CrossRef]
- Posfai, M.; Buseck, P.R. Djurleite, digenite, and chalcocite: Intergrowths and transformations. Am. Mineral. 1994, 79, 308–315. [Google Scholar]










| Sample ID | Density (g/cm3) | Standard Error (SE) | Relative Density |
|---|---|---|---|
| Printed Part | 2.79 | 0.04 | 49.9 |
| Sintered | 2.84 | 0.03 | 50.8 |
| Post-Processed | 2.68 | 0.07 | 47.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gustinvil, R.; Wright, W.J.; Di Benedetto, G.L.; Skelton, D.; Stuart, S.; Drazin, J.W.; Celik, E. Enhancing Conversion Efficiency of Direct Ink Write Printed Copper (I) Sulfide Thermoelectrics via Sulfur Infusion Process. Machines 2023, 11, 881. https://doi.org/10.3390/machines11090881
Gustinvil R, Wright WJ, Di Benedetto GL, Skelton D, Stuart S, Drazin JW, Celik E. Enhancing Conversion Efficiency of Direct Ink Write Printed Copper (I) Sulfide Thermoelectrics via Sulfur Infusion Process. Machines. 2023; 11(9):881. https://doi.org/10.3390/machines11090881
Chicago/Turabian StyleGustinvil, Raden, William Jordan Wright, Giuseppe L. Di Benedetto, Donald Skelton, Samuel Stuart, John W. Drazin, and Emrah Celik. 2023. "Enhancing Conversion Efficiency of Direct Ink Write Printed Copper (I) Sulfide Thermoelectrics via Sulfur Infusion Process" Machines 11, no. 9: 881. https://doi.org/10.3390/machines11090881