Biorefinery of Sewage Sludge: Overview of Possible Value-Added Products and Applicable Process Technologies
Abstract
:1. Introduction
2. Sewage Sludge Characteristics
3. Principles of Biorefinery
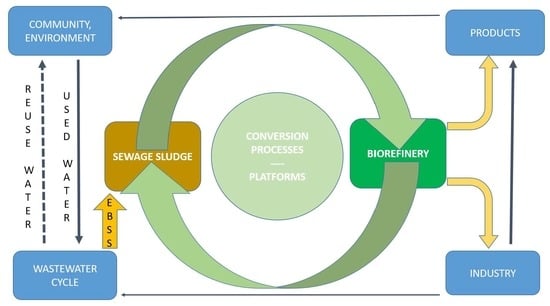
- Platforms: the intermediates that connect different biorefinery systems to their processes. The number of involved platforms is an indication of system complexity;
- Products: the many possible final outcomes of biorefinery. Two possible product groups are energy (e.g., bioethanol, biodiesel, synthetic biofuels, etc.) and material products (e.g., chemicals, materials, food, and feed);
- Feedstocks: the starting materials on which biorefinery is based. They can be grouped as either energy crops from agriculture and forestry, biomass residues from agriculture, forestry, trade, and industry, or specific organic waste streams. The latter includes sewage sludge;
- Conversion processes: the main four process groups include biochemical (e.g., fermentation and enzymatic), thermochemical (e.g., gasification and pyrolysis), chemical (e.g., acid hydrolysis, synthesis, and esterification), and mechanical processes (e.g., fractionation, pressing, and size reduction).
4. Sewage Sludge Biorefinery
4.1. Raw Chemicals Recovery from EBSS
4.1.1. Central Role of Anaerobic Fermentation in EBSS Exploitation
4.1.2. Biopolymers
4.1.3. Proteins
4.1.4. Added-Value Products from Biological Processing of EBSS
4.1.5. Added-Value Products from the Chemical Processing of EBSS
5. Discussion
6. Conclusions
- Continuing product innovation: integrated, new urban biorefinery approaches could increase the range of possible final products and their economic and environmental value. Innovative strategies should be investigated for the development of viable sludge biorefineries;
- Marketability within a circular economy approach: biohydrogen production from the AD of carbon-rich sludge is limited by low yield and VFA accumulation, making the process uncompetitive. Protein and enzymes still present higher production costs compared to traditional sources; concerns about animal health still persist in terms of pathogens and metals content; the purification of recovered bioproducts should be further developed to comply with requirements for specific applications to improve their economic added value;
- Process technology: the recovery of metabolic products (e.g., bioplastics, biopesticides, bioflocculants, and biosurfactants) and EBSS postprocessing such as fermentation and enzymatic extraction still require significant TRL optimisation, including scaling-up to pilot or full industrial size; production and extraction of SCVFAs via anaerobic digestion should be further developed;
- Sustainability, social acceptance, and regulatory framework: each scenario needs specific evaluation in terms of yield and composition of feedstocks. The development of assessment tools for processes/product integration and optimization should be promoted. The integration of regulatory instruments, the elimination of legislative barriers, and efforts aimed at improving consumer attitudes could offer important incentives towards the long-term viability of biorefinery schemes.
Funding
Data Availability Statement
Conflicts of Interest
References
- Sludgetreat. Eco-Friendly and Energy Efficient Sewage SLUDGE dewaTeRing through Novel Nanomaterials and Electro-Osmotic Process. Project Co-Funded by the European Commission within the FP7 (2007–2013) Marie Curie Actions—Industry-Academia Partnerships and Pathways (IAPP). 2014. Available online: https://sludgetreat.eu/wp-content/uploads/2016/11/D2.2-Preliminary-market-analysis-review1.pdf (accessed on 11 November 2022).
- University of Michigan. U.S. Wastewater Treatment—Factsheet Water. Center for Sustainable Systems. 2021. Available online: https://css.umich.edu/sites/default/files/Wastewater%20Treatment_CSS04-14_e2021.pdf (accessed on 11 November 2022).
- Wei, L.; Zhu, F.; Lia, Q.; Xue, C.; Xia, X.; Yu, H.; Zhao, Q.; Jiang, J.; Bai, B. Development, current state and future trends of sludge management in China: Based on exploratory data and CO2-equivaient emissions analysis. Environ. Int. 2020, 144, 106093. [Google Scholar] [CrossRef]
- Dubey, M.; Mohapatra, S.; Tyagi, V.K.; Suthar, S.; Kazmi, A.A. Occurrence, fate, and persistence of emerging micropollutants in sewage sludge treatment. Environ. Pollut. 2021, 273, 116515. [Google Scholar] [CrossRef] [PubMed]
- Sivaramakrishnan, S. 120,000 tonnes of Faecal Sludge: Why India Needs a Market for Human Waste. World Econ. Forum. 2019. Available online: https://www.weforum.org/agenda/2019/09/how-to-improve-sanitation-in-india/#:~:text=India’s%20urban%20areas%20produce%20120%2C000,connected%20to%20the%20sewer%20system (accessed on 9 December 2022).
- Battista, F.; Frison NPavan, P.; Cavinato, C.; Gottardo, M.; Fatone, F.; Eusebi, A.L.; Majone, M.; Zeppilli, M.; Valentino, F.; Fino, D.; et al. Food wastes and sewage sludge as feedstock for an urban biorefinery producing biofuels and added-value bioproducts. J. Chem. Technol. Biotechnol. 2020, 95, 328–338. [Google Scholar] [CrossRef]
- Trojanowicz, M.; Bojanowska-Czajka, A.; Capodaglio, A.G. Can radiation chemistry supply a highly efficient AO(R)P process for organics removal from drinking and waste water? A review. Environ. Sci. Pollut. Res. 2017, 24, 20187–20208. [Google Scholar] [CrossRef] [PubMed]
- EurEau. Waste Water Treatment—Sludge Management—Briefing Note 2021. Available online: https://www.eureau.org/resources/briefing-notes/5629-briefing-note-on-sludge-management/file (accessed on 11 December 2022).
- Capodaglio, A.G.; Olsson, G. Energy issues in sustainable urban wastewater management: Use, demand reduction and recovery in the urban water cycle. Sustainability 2020, 12, 266. [Google Scholar] [CrossRef] [Green Version]
- Kacprzak, M.; Neczaj, E.; Fijałkowski, K.; Grobelak, A.; Grosser, A.; Worwag, M.; Rorata, A.; Brattebob, H.; Almåsc, Å.; Singh, B.R. Sewage sludge disposal strategies for sustainable development. Environ. Res. 2017, 156, 39–46. [Google Scholar] [CrossRef]
- Angelakis, A.N.; Capodaglio, A.G.; Dialynas, E.G. Wastewater Management: From Ancient Greece to Modern Times and Future. Water 2023, 15, 43. [Google Scholar] [CrossRef]
- Hudcová, H.; Vymazal, J.; Rozkošný, M. Present restrictions of sewage sludge application in agriculture within the European Union. Soil Water Res. 2019, 14, 104–120. [Google Scholar] [CrossRef] [Green Version]
- Boguniewicz-Zablocka, J.; Klosok-Bazan, I.; Capodaglio, A.G. Sustainable management of biological solids in small treatment plants: Overview of strategies and reuse options for a solar drying facility in Poland. Environ. Sci. Pollut. Res. 2021, 28, 24680–24693. [Google Scholar] [CrossRef]
- Eurostat. Guidance on Classification of Waste according to EWC-Stat Categories. Supplement to the Manual for the Implementation of the Regulation (EC) No 2150/2002 on Waste Statistic; Commission of The European Communities, Directorate E: Sectoral and regional statistics; Eurostat: Brussels, Belgium, 2010. [Google Scholar]
- Water Europe. Public Consultation on the “Sewage Sludge Use in Farming” Directive is Now Open. 2020. Available online: https://watereurope.eu/public-consultation-of-sewage-sludge-use-in-farming-directive-is-now-open/ (accessed on 27 October 2022).
- Capodaglio, A.G. Fit-for-purpose urban wastewater reuse: Analysis of issues and available technologies for sustainable multiple barrier approaches. Crit. Rev. Environ. Sci. Technol. 2021, 51, 1619–1666. [Google Scholar] [CrossRef]
- Daneshgar, S.; Buttafava, A.; Callegari, A.; Capodaglio, A.G. Economic and energetic assessment of different phosphorus recovery options from aerobic sludge. J. Clean. Prod. 2019, 223, 729–738. [Google Scholar] [CrossRef]
- Cecconet, D.; Raček, J.; Callegari, A.; Hlavínek, P. Energy Recovery from Wastewater: A Study on Heating and Cooling of a Multipurpose Building with Sewage-Reclaimed Heat Energy. Sustainability 2020, 12, 116. [Google Scholar] [CrossRef] [Green Version]
- Appels, L.; Baeyens, J.; Degreve, J.; Dewil, R. Principles and potential of the anaerobic digestion of waste-activated sludge. Prog. Energy Combust. 2008, 34, 755–781. [Google Scholar] [CrossRef]
- Callegari, A.; Hlavinek, P.; Capodaglio, A.G. Production of energy (biodiesel) and recovery of materials (biochar) from pyrolysis of urban waste sludge. Rev. Ambiente Água 2018, 13, e2128. [Google Scholar] [CrossRef]
- Daneshgar, S.; Vanrolleghem, P.A.; Vaneeckhaute, C.; Buttafava, A.; Capodaglio, A.G. Optimization of P compounds recovery from aerobic sludge by chemical modeling and response surface methodology combination. Sci. Total Environ. 2019, 668, 668–677. [Google Scholar] [CrossRef]
- Crutchik, D.; Franchi, O.; Caminos, L.; Jeison, D.; Belmonte, M.; Pedrouso, A.; Val del Rio, A.; Mosquera-Corral, A.; Campos, J.L. Polyhydroxyalkanoates (PHAs) Production: A Feasible Economic Option for the Treatment of Sewage Sludge in Municipal Wastewater Treatment Plants? Water 2020, 12, 1118. [Google Scholar] [CrossRef]
- Li, J.; Hao, X.D.; Gan, W.; van Loosdrecht, M.C.M.; Wu, Y.Y. Recovery of extracellular biopolymers from conventional activated sludge: Potential, characteristics and limitation. Water Res. 2021, 205, 117706. [Google Scholar] [CrossRef]
- Shizas, I.; Bagley, D.M. Experimental determination of energy content of unknown organics in municipal wastewater streams. J. Energy Eng. 2004, 130, 45–53. [Google Scholar] [CrossRef]
- Tchobanoglous, G.; Barton, F.; Stensel, H. Metcalf and Eddy’s Wastewater Engineering Treatment and Reuse, 4th ed.; McGraw Hill: New York, NY, USA, 2003. [Google Scholar]
- Spinosa, L. Standardized characterization procedures: A necessary support to regulations. Water Sci. Technol. 2016, 74, 220–228. [Google Scholar] [CrossRef]
- National Biosolids Partnership. National Manual of Good Practice for Biosolids; Water Environment Research Foundation: Alexandria, VA, USA, 2011. [Google Scholar]
- EC, Sewage Sludge. European Commission Directorate-General for Environment. 2022. Available online: https://environment.ec.europa.eu/topics/waste-and-recycling/sewage-sludge_en#publications (accessed on 7 December 2022).
- Li, J.; Luo, G.; Xu, J. Fate and Ecological Risk Assessment of Nutrients and Metals in Sewage Sludge from Ten Wastewater Treatment Plants in Wuxi City, China. Bull. Environ. Contam. Toxicol. 2019, 102, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Corradini, F.; Meza, P.; Eguiluz, R.; Casado, F.; Huerta-Lwang, E.; Geissen, V. Evidence of microplastic accumulation in agricultural soils from sewage sludge disposal. Sci. Total Environ. 2019, 671, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Hennebert, P.; Anderson, A.; Merdy, P. Mineral Nanoparticles in Waste: Potential Sources, Occurrence in Some Engineered Nanomaterials Leachates, Municipal Sewage Sludges and Municipal Landfill Sludges. J. Biotechnol. Biomater. 2017, 7, 261. [Google Scholar] [CrossRef]
- Fijalkowski, K. Emerging contaminants in sludge (endocrine disruptors, pesticides, and pharmaceutical residues, including illicit drugs/controlled substances, etc.). In Industrial and Municipal Sludge Emerging Concerns and Scope for Resource Recovery; Butterworth: Oxford, UK, 2019; pp. 455–473. [Google Scholar]
- Rosinska, A. Traditional contaminants in sludge. In Industrial and Municipal Sludge Emerging Concerns and Scope for Resource Recovery; Butterworth: Oxford, UK, 2019; pp. 426–453. [Google Scholar]
- Mailler, R.; Gasperi, J.; Patureau, D.; Vulliet, E.; Delgenes, N.; Danel NDeshayesa, S.; Eudes, V.; Guerine, S.; Moilleron, R.; Chebbo, G.; et al. Fate of emerging and priority micropollutants during the sewage sludge treatment: Case study of Paris conurbation. Part 1: Contamination of the different types of sewage sludge. Waste Manag. 2017, 59, 379–393. [Google Scholar] [CrossRef] [Green Version]
- Huang, K.; Mao, Y.; Zhao, F.; Zhang, X.-X.; Ju, F.; Ye, L.; Wang, Y.; Li, B.; Ren, H.; Zhang, T. Free-living bacteria and potential bacterial pathogens in sewage treatment plants. Appl. Microbiol. Biotechnol. 2018, 102, 2455–2464. [Google Scholar] [CrossRef]
- Tyagi, V.K.; Lo, S.L. Sludge: A waste or renewable source for energy and resources recovery? Renew. Sustain. Energy Rev. 2013, 25, 708–728. [Google Scholar] [CrossRef]
- Keiding, K.; Wybrandt, L.; Nielsen, P.H. Remember the water: A comment on EPS colligative properties. Water Sci. Technol. 2001, 43, 17–23. [Google Scholar] [CrossRef]
- Ding, Z.; Bourven, I.; Guibaud, G.; van Hullebusch, E.D.; Panico, A.; Pirozzi, F.; Esposito, G. Role of extracellular polymeric substances (EPS) production in bioaggregation: Application to wastewater treatment. Appl. Microbiol. Biotechnol. 2015, 99, 9883–9905. [Google Scholar] [CrossRef]
- Feng, C.; Lotti, T.; Canziani, R.; Lin, Y.; Tagliabue, C.; Malpei, F. Extracellular biopolymers recovered as raw biomaterials from waste granular sludge and potential applications: A critical review. Sci. Total Environ. 2021, 753, 142051. [Google Scholar] [CrossRef]
- BIOPOL. 2009. Available online: http://www.biorenery.nl/leadmin/biopol/user/documents/PublicDeliverables/BIOPOL_D_7_6_-_Final_240609.pdf (accessed on 27 October 2022).
- IEA. Technical, Economic and Environmental Assessment of Biorefinery Concept; IEA Bioenergy: Paris, France, 2019; ISBN 978-1-910154-64-9. [Google Scholar]
- Cherubini, F.; Jungmeier, G.; Wellisch, M.; Wilke, T.; Skiadas, I.; Ree, V.R.; Jong, D.E. Toward a common classification approach for biorefinery systems. Biofuels Bioprod. Biorefining 2009, 3, 534–546. [Google Scholar] [CrossRef]
- Nizami, A.S.; Rehan, N.; Waqas, M.; Naqvi, M.; Ouda, O.K.M.; Shahzad, K.; Miandad, R.; Khan, M.Z.; Syamsiro, M.; Ismail, I.M.I.; et al. Waste biorefineries: Enabling circular economies in developing countries. Bioresour. Technol. 2017, 241, 1101–1117. [Google Scholar] [CrossRef] [PubMed]
- Capodaglio, A.G.; Bolognesi, S.; Cecconet, D. Sustainable, decentralized sanitation and reuse with hybrid nature-based systems. Water 2021, 13, 1583. [Google Scholar] [CrossRef]
- Li, K.; Liu, Q.; Fang, F.; Luo, R.; Lu, Q.; Zhou, W.; Huo, S.; Cheng, P.; Liu, J.; Addy, M.; et al. Microalgae-based wastewater treatment for nutrients recovery: A review. Bioresour. Technol. 2019, 291, 121934. [Google Scholar] [CrossRef]
- Bolognesi, S.; Bernardi, G.; Callegari, A.; Dondi, D.; Capodaglio, A.G. Biochar production from sewage sludge and microalgae mixtures: Properties, sustainability and possible role in circular economy. Biomass Convers. Biorefinery 2021, 11, 289–299. [Google Scholar] [CrossRef]
- Bolognesi, S.; Bañeras, L.; Perona-Vico, E.; Capodaglio, A.G.; Balaguer MDPuig, S. Carbon dioxide to bio-oil in a bioelectrochemical system-assisted microalgae biorefinery process. Sustain. Energy Fuels 2022, 6, 150–161. [Google Scholar] [CrossRef]
- Callegari, A.; Bolognesi, S.; Cecconet, D.; Capodaglio, A.G. Production technologies, current role, and future prospects of biofuels feedstocks: A state-of-the-art review. Crit. Rev. Environ. Sci. Technol. 2020, 50, 384–436. [Google Scholar] [CrossRef]
- Tenenbaum, D.J. Food vs. fuel: Diversion of crops could cause more hunger. Environ. Health Perspect. 2008, 116, A254–A257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bora, R.R.; Richardson, R.E.; You, F. Resource recovery and waste-to-energy from wastewater sludge via thermochemical conversion technologies in support of circular economy: A comprehensive review. BMC Chem. Eng. 2020, 2, 8. [Google Scholar] [CrossRef]
- Gao, N.; Kamran, K.; Quan, C.; Williams, P.T. Thermochemical conversion of sewage sludge: A critical review. Prog. Energy Combust. Sci. 2020, 79, 100843. [Google Scholar] [CrossRef]
- Hu, M.; Ye, Z.; Zhang, H.; Chen, B.; Pan, Z.; Wang, J. Thermochemical conversion of sewage sludge for energy and resource recovery: Technical challenges and prospects. Environ. Pollut. Bioavailab. 2021, 33, 145–163. [Google Scholar] [CrossRef]
- Shatir, S.; Syed-Hassan, A.; Wang, Y.; Hua, S.; Su, S.; Xiang, J. Thermochemical processing of sewage sludge to energy and fuel: Fundamentals, challenges and considerations. Renew. Sustain. Energy Rev. 2017, 80, 888–913. [Google Scholar]
- Capodaglio, A.G.; Callegari, A.; Dondi, D. Microwave-Induced Pyrolysis for Production of Sustainable Biodiesel from Waste Sludges. Waste Biom. Valoriz 2016, 7, 703–709. [Google Scholar] [CrossRef]
- Ledakowicz, S.; Stolarek, P.; Malinowski, A.; Lepez, O. Thermochemical treatment of sewage sludge by integration of drying and pyrolysis/autogasification. Renew. Sustain. Energy Rev. 2019, 104, 319–327. [Google Scholar] [CrossRef]
- Wang, X.; Chi, Q.; Liu, X.; Wang, Y. Influence of pyrolysis temperature on characteristics and environmental risk of heavy metals in pyrolyzed biochar made from hydrothermally treated sewage sludge. Chemosphere 2019, 216, 698–706. [Google Scholar] [CrossRef]
- Capodaglio, A.G.; Callegari, A.; Lopez, M.V. European Framework for the Diffusion of Biogas Uses: Emerging Technologies, Acceptance, Incentive Strategies, and Institutional-Regulatory Support. Sustainability 2016, 8, 298. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zhao, J.; Wang, D.; Yang, Q.; Zeng, G. An efficient and green pretreatment to stimulate short-chain fatty acids production from waste activated sludge anaerobic fermentation using free nitrous acid. Chemosphere 2016, 144, 160–167. [Google Scholar] [CrossRef]
- Zhang, C.; Qin, Y.; Xu, Q.; Liu, X.; Liu, Y.; Ni, B.J.; Yang, Q.; Wang, D.; Li, X.; Wang, Q. Free ammonia-based pretreatment promotes short-chain fatty acid production from waste activated sludge. ACS Sustain. Chem. Eng. 2018, 6, 9120–9129. [Google Scholar] [CrossRef]
- Liu, H.; Fang, H.H. Extraction of extracellular polymeric substances (EPS) of sludges. J. Biotechnol. 2002, 95, 249–256. [Google Scholar] [CrossRef]
- Cord-Ruwisch, R. Thermodynamics of anaerobic digestion: Mechanism of suppression on biogas production during acidogenesis. INMATEH Agric. Eng. 2019, 57, 287–301. [Google Scholar]
- Shen, L.; Hu, H.; Ji, H.; Cai, J.; He, N.; Li, Q.; Wang, Y. Production of poly(hydroxybutyrate-hydroxyvalerate) from waste organics by the two-stage process: Focus on the intermediate volatile fatty acids. Biores Technol. 2014, 166, 194–200. [Google Scholar] [CrossRef]
- Levy, P.F.; Sanderson, J.E.; Kispert, R.G.; Wise, D.L. Biorefining of biomass to liquid fuels and organic chemicals. Enzym. Microb. Technol. 1981, 3, 207–215. [Google Scholar] [CrossRef]
- Atasoy, M.; Owusu-Agyeman, I.; Plaza, E.; Cetecioglu, Z. Bio-based volatile fatty acid production and recovery from waste streams: Current status and future challenges. Bioresour. Technol. 2018, 268, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Owusu-Agyeman, I.; Plaza, E.; Cetecioglu, Z. Production of volatile fatty acids through co-digestion of sewage sludge and external organic waste: Effect of substrate proportions and long-term operation. Waste Manag. 2020, 112, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Zacharof, M.P.; Lovitt, R.W. Complex effluent streams as a potential source of volatile fatty acids. Waste Biomass Valorization 2013, 4, 557–581. [Google Scholar] [CrossRef]
- Wu, S.L.; Wei, W.; Sun, J.; Xu, Q.; Dai, X.; Ni, B.J. Medium-Chain fatty acids and long-chain alcohols production from waste activated sludge via two-stage anaerobic fermentation. Water Res. 2020, 186, 116381. [Google Scholar] [CrossRef]
- Yishai, O.; Lindner, S.N.; de la Cruz, J.G.; Tenenboim, H.; Bar-Even, A. The formate bio-economy. Curr. Opin. Chem. Biol. 2016, 35, 1–9. [Google Scholar] [CrossRef]
- Wang, R.; Lv, N.; Li, C.; Cai, G.; Pan, X.; Li, Y.; Zhu, G. Novel strategy for enhancing acetic and formic acids generation in acidogenesis of anaerobic digestion via targeted adjusting environmental niches. Water Res. 2021, 193, 116896. [Google Scholar] [CrossRef]
- Mühlemeier, I.M.; Speight, R.; Strong, P.J. Biogas, bioreactors and bacterial methane oxidation. In Methane Biocatalysis: Paving the Way to Sustainability; Kalyuzhnaya, M.G., Xing, X.-H., Eds.; Springer International Publishing AG: Berlin/Heidelberg, Germany, 2018; pp. 213–235. [Google Scholar]
- Zhang, T.; Zhou, J.; Wang, X.; Zhang, Y. Coupled effects of methane monooxygenase and nitrogen source on growth and poly-β-hydroxybutyrate (PHB) production of Methylosinus trichosporium OB3b. J. Environ. Sci. 2017, 52, 49–57. [Google Scholar] [CrossRef]
- Sander, R. Compilation of Henry’s law constants (version 4.0) for water as solvent. Atmos. Chem. Phys. 2015, 15, 4399–4981. [Google Scholar] [CrossRef] [Green Version]
- Cantera, S.; Estrada, J.M.; Lebrero, R.; García-Encina, P.A.; Muñoz, R. Comparative performance evaluation of conventional and two-phase hydrophobic stirred tank reactors for methane abatement: Mass transfer and biological considerations. Biotechnol. Bioeng. 2016, 113, 1203–1212. [Google Scholar] [CrossRef]
- Rodríguez, Y.; Firmino, P.I.M.; Pérez, V.; Lebrero, R.; Muñoz, R. Biogas valorization via continuous polyhydroxybutyrate production by Methylocystis hirsuta in a bubble column bioreactor. Waste Manag. 2020, 113, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Chen, J.C.; Wu, Q.; Chen, G.Q. Polyhydroxyalkanoates as a source of chemicals, polymers, and biofuels. Curr. Opin. Biotechnol. 2011, 22, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Spekreijse, J.; Le Nôtre, J.; van Haveren, J.; Scott, E.L.; Sanders, J.P.M. Simultaneous production of biobased styrene and acrylates using ethenolysis. Green Chem. 2014, 14, 2747–2751. [Google Scholar] [CrossRef]
- Bluemink, E.D.; van Nieuwenhuijzen, A.F.; Wypkema, E.; Uijterlinde, C.A. Bio-plastic (poly-hydroxy-alkanoate) production from municipal sewage sludge in the Netherlands: A technology push or a demand driven process? Water Sci. Technol. 2016, 74, 353–358. [Google Scholar] [CrossRef] [Green Version]
- Yadav, B.; Talan, A.; Tyagi, R.D.; Drogui, P. Concomitant production of value-added products with polyhydroxyalkanoate (PHA) synthesis: A review. Bioresour. Technol. 2021, 337, 125419. [Google Scholar] [CrossRef]
- Morgan-Sagastume, F.; Hjort, M.; Cirne, D.; Gérardin, F.; Lacroix, S.; Gaval, G.; Karabegovic, L.; Alexandersson, T.; Johansson, P.; Karlsson, A.; et al. Integrated production of polyhydroxyalkanoates (PHAs) with municipal wastewater and sludge treatment at pilot scale. Bioresour. Technol. 2015, 181, 78–89. [Google Scholar] [CrossRef]
- Agler, M.T.; Wrenn, B.A.; Zinder, S.H.; Angenent, L.T. Waste to bioproduct conversion with undefined mixed cultures: The carboxylate platform. Trends Biotechnol. 2011, 29, 70–78. [Google Scholar] [CrossRef]
- Valentino, F.; Morgan-Sagastume, F.; Fraraccio, S.; Corsi, G.; Zanaroli, G.; Werker, A.; Majone, M. Sludge minimization in municipal wastewater treatment by polyhydroxyalkanoate (PHA) production. Environ. Sci. Pollut. Res. 2015, 22, 7281–7294. [Google Scholar] [CrossRef]
- Frison, N.; Katsou, E.; Malamis, S.; Oehmen, A.; Fatone, F. Development of a novel process integrating the treatment of sludge reject water and the production of polyhydroxyalkanoates (PHAs). Environ.Sci. Technol. 2015, 49, 10877–10885. [Google Scholar] [CrossRef] [Green Version]
- Serafim, L.S.; Lemos, P.C.; Albuquerque, M.G.E.; Reis, M.A.M. Strategies for PHA production by mixed cultures and renewable waste materials. Appl. Microbiol. Biotechnol. 2008, 81, 615–628. [Google Scholar] [CrossRef]
- Serafim, L.S.; Lemos, P.C.; Oliveira, R.F.; Reis, M.A.M. Optimisation of polyhydroxybutyrate production by mixed cultures submitted to aerobic dynamic feeding conditions. Biotechnol. Bioeng. 2004, 87, 145–160. [Google Scholar] [CrossRef]
- Takabatake, H.; Satoh, H.; Mino, T.; Matsuo, T. Recovery of biodegradable plastics from activated sludge process. Water Sci. Technol. 2000, 42, 351–356. [Google Scholar] [CrossRef]
- Albuquerque, M.G.E.; Martino, V.; Pollet, E.; Avérous, L.; Reis, M.A.M. Mixed culture polyhydroxyalkanoate (PHA) production from volatile fatty acid (VFA)-rich streams: Effect of substrate composition and feeding regime on PHA productivity, composition and properties. J. Biotechnol. 2011, 151, 66–76. [Google Scholar] [CrossRef]
- Baetens, D.; Aurola, A.M.; Foglia, A.; Dionisi, D.; van Loosdrecht, M.C.M. Gas chromatographic analysis of polyhydroxybutyrate in activated sludge: A round-robin test. Water Sci. Technol. 2002, 46, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Morgan-Sagastume, F.; Karlsson, A.; Johansson, P.; Pratt, S.; Boon, N.; Lant, P.; Werker, A. Production of polyhydroxyalkanoates in open, mixed cultures from a waste sludge stream containing high levels of soluble organics, nitrogen and phosphorus. Water Res. 2010, 44, 5196–5211. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. Relevance of microbial extracellular polymeric substances (EPSs) Part II: Technical aspects. Water Sci. Technol. 2001, 43, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.K.; Mao, N.; Lin, R.; Bhattacharyya, D.; van Loosdrecht, M.C.M.; Lin, Y. Flame retardant property of flax fabrics coated by extracellular polymeric substances recovered from both activated sludge and aerobic granular sludge. Water Res. 2020, 170, 115344. [Google Scholar] [CrossRef]
- Schlekat, C.E.; Decho, A.W.; Chandler, G.T. Sorption of cadmium to bacterial extracellular polymeric sediment coatings under estuarine conditions. Environ. Toxicol. Chem. 1998, 17, 1867–1874. [Google Scholar] [CrossRef]
- Mohapatra, S.P.; Siebel, M.A.; Alaerts, G.J. Effect of Bacillus megaterium on removal of copper from aqueous solutions by activated carbon. J. Environ. Sci. Health Part A Environ. Sci. Eng. Tox. Haz. Subst. Contr. 1993, 28, 615–629. [Google Scholar]
- Spath, R.; Flemming, H.C.; Wuertz, S. Sorption properties of biofilms. Water Sci. Technol. 1998, 37, 207–210. [Google Scholar] [CrossRef]
- Liu, A.B.; Ahn, I.S.; Mansfield, C.; Lion, L.W.; Shuler, M.L.; Ghiorse, W.C. Phenanthrene desorption from soil in the presence of bacterial extracellular polymer: Observations and model predictions of dynamic behaviour. Water Res. 2000, 35, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Esparza-Soto, M.; Westerhoff, P. Biosorption of humic and fulvic acids to live activated sludge biomass. Water Res. 2003, 37, 2301–2310. [Google Scholar] [CrossRef]
- Sheng, G.P.; Zhang, M.L.; Yu, H.Q. Characterization of adsorption properties of extracellular polymeric substances (EPS) extracted from sludge. Colloids Surf. B 2008, 62, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Q.; Bishop, P.L. Biodegradability of biofilm extracellular polymeric substances. Chemosphere 2003, 50, 63–69. [Google Scholar] [CrossRef]
- Andreoli, C.V.; Von Sperling, M.; Fernandes, F.; Ronteltap, M. Sludge Treatment and Disposal; IWA Publishing: London, UK, 2007. [Google Scholar]
- Lackner, S.; Gilbert, E.M.; Vlaeminck, S.E.; Joss, A.; Horn, H.; van Loosdrecht, M.C.M. Full-scale partial nitritation/anammox experiences–an application survey. Water Res. 2014, 55, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Dubé, C.D.; Guiot, S.R. Characterization of the protein fraction of the extracellular polymeric substances of three anaerobic granular sludges. AMB Express 2019, 9, 23. [Google Scholar] [CrossRef] [Green Version]
- Seviour, T.; Donose, B.C.; Pijuan, M.; Yuan, Z. Purification and conformational analysis of a key exopolysaccharide component of mixed culture aerobic sludge granules. Environ. Sci. Technol. 2010, 44, 4729–4734. [Google Scholar] [CrossRef]
- Capodaglio, A.G. Pulse electric field technology for wastewater and biomass residues’ improved valorization. Processes 2021, 9, 736. [Google Scholar] [CrossRef]
- Gehr, R.; Henry, J. Removal of extracellular material techniques and pitfalls. Water Res. 1983, 17, 1743–1748. [Google Scholar] [CrossRef]
- Felz, S.; Al-Zuhairy, S.; Aarstad, O.A.; van Loosdrecht, M.C.M.; Lin, Y.M. Extraction of structural extracellular polymeric substances from aerobic granular sludge. JOVE-J. Vis. Exp. 2016, 115, e54534. [Google Scholar]
- Frølund, B.; Palmgren, R.; Keiding, K.; Nielsen, P.H. Extraction of extracellular polymers from activated sludge using a cation exchange resin. Water Res. 1996, 30, 1749–1758. [Google Scholar] [CrossRef]
- Sheng, G.; Yu, H.; Li, X. Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: A review. Biotechnol. Adv. 2010, 28, 882–894. [Google Scholar] [CrossRef] [PubMed]
- Boleij, M.; Seviour, T.; Wong, L.L.; van Loosdrecht, M.C.M.; Lin, Y. Solubilization and characterization of extracellular proteins from anammox granular sludge. Water Res. 2019, 164, 114952. [Google Scholar] [CrossRef] [PubMed]
- Sesay, M.L.; Özcengiz, G.; Sanin, F.D. Enzymatic extraction of activated sludge extracellular polymers and implications on bioflocculation. Water Res. 2006, 40, 1359–1366. [Google Scholar] [CrossRef]
- Markham, W.M.; Reid, J.H. Conversion of Biological Sludge and Primary Float Sludge to Animal Protein Supplement. U.S. Patent No 4,728,517, 1 March 1988. [Google Scholar]
- Rudolf, P.; Szabo, B.; Janko, F.; Neszmelyi, E.; Illes, J.; Takacs, I.; Havas, F.; Bende, G. Process and Apparatus for Extraction of Solid Matter Containing Fat And/or Protein from Sludge. US Patent 5200085A, 6 April 1993. [Google Scholar]
- Pervaiz, M.; Sain, M. Protein extraction from secondary sludge of paper mill wastewater and its utilization as a wood adhesive. Bioresources 2021, 6, 961–970. [Google Scholar]
- Zhu, H. Study on preparation of protein foam fire extinguishing agent. Special. Petrochem. 1994, 1, 1–7. [Google Scholar]
- Hwang, J.; Zhang, L.; Seo, S.; Lee, Y.W.; Jahng, D. Protein recovery from excess sludge for its use as animal feed. Bioresour. Technol. 2008, 99, 8949–8954. [Google Scholar] [CrossRef]
- Xiao, K.; Zhou, Y. Protein recovery from sludge: A review. J. Clean. Prod. 2020, 249, 119373. [Google Scholar] [CrossRef]
- Ayol, A. Enzymatic treatment effects on dewaterability of anaerobically digested biosolids-I: Performance evaluations. Process Biochem. 2005, 40, 2427–2434. [Google Scholar] [CrossRef]
- Su, R.; Hussain, A.; Guo, J.; Guan, J.; He, Q.; Yan, X.; Li, D.; Guo, Z. Animal feeds extracted from excess sludge by enzyme, acid and base hydrolysis processes. ACS Sust. Chem. Eng. 2015, 3, 2084–2091. [Google Scholar] [CrossRef]
- Kavitha, S.; Stella, P.C.; Kaliappan, S.; Yeom, I.T.; Banu, J.R. Enhancement of anaerobic degradation of sludge biomass through surfactant-assisted bacterial hydrolysis. Process Saf. Environ. Prot. 2016, 99, 207–215. [Google Scholar] [CrossRef]
- Zhuang, L.; Zhou, S.; Wang, Y.; Liu, Z.; Xu, R. Cost-effective production of Bacillus thuringiensis biopesticides by solid-state fermentation using wastewater sludge: Effects of heavy metals. Bioresour. Technol. 2011, 102, 4820–4826. [Google Scholar] [CrossRef] [PubMed]
- Brar, S.K.; Verma, M.; Tyagi, R.D.; Valéro, J.R.; Surampalli, R.Y. Biopesticides—Bacillus thuringiensis. In Sustainable Sludge Management: Production of Value Added Products; Tyagi, R.D., Surampalli, R.Y., Yan, S., Zhang, T.C., Kao, C.M., Lohani, B.N., Eds.; American Society of Civil Engineers: Reston, VA, USA, 2013; pp. 168–202. [Google Scholar]
- Montiel, M.T.; Tyagi, R.; Valero, J.; Surampalli, R. Production of biopesticides using wastewater sludge as a raw material-effect of process parameters. Water Sci. Technol. 2003, 48, 239–246. [Google Scholar] [CrossRef]
- Drouin, M.; Lai, C.K.; Tyagi, R.D.; Surampalli, R.Y. Bacillus licheniformis proteases as high value added products from fermentation of wastewater sludge: Pre-treatment of sludge to increase the performance of the process. In Proceedings of the IWA Specialist Conference on Moving Forward Wastewater Biosolids Sustainability: Technical, Managerial, and Public Synergy, Moncton, NB, Canada, 24–27 June 2007; pp. 599–605. [Google Scholar]
- Balasubramanian, S.; Tyagi, R.D. Value-Added Bio-products From Sewage Sludge. In Current Developments in Biotechnology and Bioengineering; Wong, J.-C., Tyagi, R., Pandey, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 27–42. [Google Scholar]
- Frølund, B.; Palmgren, R.; Keiding, K.; Nielsen, P.H. Enzymatic activity in the activated sludge floc matrix. Appl. Microbiol. Biotechnol. 1995, 43, 708–716. [Google Scholar] [CrossRef]
- Subramanian, S.B.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Enzymes: Production and Extraction. In Sustainable Sludge Management: Production of Value Added Products; Tyagi, R.D., Surampalli, R.Y., Yan, S., Zhang, T.C., Kao, C.M., Lohani, B.N., Eds.; American Society of Civil Engineers: Reston, VA, USA, 2013; pp. 231–261. [Google Scholar]
- Feng, S.; Ngo, H.H.; Guo, W.; Chang, S.W.; Nguyen, D.D.; Cheng, D.; Varjani SLei, Z.; Liu, Y. Roles and applications of enzymes for resistant pollutants removal in wastewater treatment. Bioresour. Technol. 2021, 335, 125278. [Google Scholar] [CrossRef] [PubMed]
- Guanghui, Y.; Pinjing, H.; Liming, S.; Yishu, Z. Enzyme extraction by ultrasound from sludge flocs. J. Environ. Sci. 2009, 21, 204–210. [Google Scholar]
- Nabarlatz, D.; Vondrysova, J.; Jenicek, P.; Stüber, F.; Font, J.; Fortuny, A.; Fabregat, A.; Bengoa, C. Hydrolytic enzymes in activated sludge: Extraction of protease and lipase by stirring and ultrasonication. Ultrason. Sonochem. 2010, 17, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Xing, X.H.; Matsumoto, K. Recoverability of protease released from disrupted excess sludge and its potential application to enhanced hydrolysis of proteins in wastewater. Biochem. Eng. J. 2002, 10, 67–72. [Google Scholar] [CrossRef]
- Petersen, S.B.; Nielsen, P.H. Lipase and protease extraction from activated sludge. Water Res. 2003, 37, 3652–3657. [Google Scholar]
- Agrafioti, E.; Bouras, G.; Kalderis, D.; Diamadopoulos, E. Biochar production by sewage sludge pyrolysis. J. Anal. Appl. Pyrolysis 2013, 101, 72–78. [Google Scholar] [CrossRef]
- Capodaglio, A.G.; Callegari, A. Feedstock and process influence on biodiesel produced from waste sewage sludge. J. Environ. Manag. 2018, 216, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Djandja, O.S.; Wang, Z.C.; Wang, F.; Xu, Y.P.; Duan, P.G. Pyrolysis of Municipal Sewage Sludge for Biofuel Production: A Review. Ind. Eng. Chem. Res. 2020, 59, 16939–16956. [Google Scholar] [CrossRef]
- Mushtaq, F.; Mat, R.; Ani, R.F. A review on microwave assisted pyrolysis of coal and biomass for fuel production. Renew. Sustain. Energy Rev. 2014, 39, 555–574. [Google Scholar] [CrossRef]
- Quan, L.M.; Kamyab, H.; Yuzir, A.; Ashokkumar, V.; Hosseini, S.E.; Balasubramanian, B.; Kirpichnikova, I. Review of the application of gasification and combustion technology and waste-to-energy technologies in sewage sludge treatment. Fuel 2022, 316, 123199. [Google Scholar] [CrossRef]
- Tasca, A.L.; Puccini, M.; Gori, R.; Corsi, I.; Galletti AM, R.; Vitolo, S. Hydrothermal carbonization of sewage sludge: A critical analysis of process severity, hydrochar properties and environmental implications. Waste Manag. 2019, 93, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, L.B.S.; Carvalho, P.N.; Dos Passos, J.S.; Anastasakis, K.; Bester, K.; Biller, P. Hydrothermal liquefaction of sewage sludge; energy considerations and fate of micropollutants during pilot scale processing. Water Res. 2020, 183, 116101. [Google Scholar] [CrossRef]
- Olkiewicz, M.; Caporgno, M.P.; Fortuny, P.; Stüber, F.; Fabregat, A.; Font, J.; Bengoa, C.M. Direct liquid-liquid extraction of lipid from municipal sewage sludge for biodiesel production. Fuel Process. Technol. 2014, 128, 331–338. [Google Scholar] [CrossRef] [Green Version]
- Boocock, D.G.B.; Konar, S.K.; Leung, A.; Ly, L.D. Fuels and chemicals from sewage sludge: 1. The solvent extraction and composition of a lipid from a raw sewage sludge. Fuel 1992, 71, 1283–1289. [Google Scholar] [CrossRef]
- Siddiquee, M.N.; Rohani, S. Lipid extraction and biodiesel production from municipal sewage sludges: A review. Renew. Sustain. Energy Rev. 2011, 15, 1067–1072. [Google Scholar] [CrossRef]
- Patiño, Y.; Mantecón, L.G.; Polo, S.; Faba, L.; Díaz, E.; Ordóñez, S. Effect of sludge features and extraction-esterification technology on the synthesis of biodiesel from secondary wastewater treatment sludges. Bioresour. Technol. 2018, 247, 209–216. [Google Scholar] [CrossRef]
- Sakaveli, F.; Petala, M.; Tsiridis, V.; Darakas, E. Enhanced mesophilic anaerobic digestion of primary sewage sludge. Water 2021, 13, 348. [Google Scholar] [CrossRef]
- D’Ambrosio, V.; di Bitonto, L.; Angelini, A.; Gallipoli ABraguglia, C.M.; Pastore, C. Lipid extraction from sewage sludge using green biosolvent for sustainable biodiesel production. J. Clean. Prod. 2021, 329, 129643. [Google Scholar] [CrossRef]
- Olkiewicz, M.; Fortuny, P.; Stüber, F.; Fabregat, A.; Font, J.; Bengoa, C. Effects of pre-treatments on the lipid extraction and biodiesel production from municipal WWTP sludge. Fuel 2015, 141, 250–257. [Google Scholar] [CrossRef] [Green Version]
- de Jesus, S.S.; Maciel Filho, R. Recent advances in lipid extraction using green solvents. Renew. Sustain. Energy Rev. 2020, 133, 110289. [Google Scholar] [CrossRef]
- Raboni, M.; Viotti, P.; Capodaglio, A.G. A comprehensive analysis of the current and future role of biofuels for transport in the European Union (EU). Rev. Ambiente Agua 2015, 10, 9–21. [Google Scholar] [CrossRef]
- Pan, J.; Ma, Y.; Zhai, L.; Luo, T.; Mei, Z.; Liu, H. Achievements of biochar application for enhanced anaerobic digestion: A review. Bioresour. Technol. 2019, 292, 122058. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Atiyeh, H.K.; Li, M.; Chen, M. Biochar facilitated bioprocessing and biorefinery for productions of biofuel and chemicals: A review. Bioresour. Technol. 2020, 295, 22252. [Google Scholar] [CrossRef]
- Teoh, S.K.; Li, L.Y. Feasibility of alternative sewage sludge treatment methods from a lifecycle assessment (LCA) perspective. J. Clean. Prod. 2020, 247, 119495. [Google Scholar] [CrossRef]
- Ding, A.; Zhang, R.; Ngo, H.H.; He, X.; Ma, J.; Nan, J.; Li, G. Life cycle assessment of sewage sludge treatment and disposal based on nutrient and energy recovery: A review. Sci. Total Environ. 2021, 769, 144451. [Google Scholar] [CrossRef]
- Mayer, F.; Bhandari, R.; Gäth, S.A. Life cycle assessment of prospective sewage sludge treatment paths in Germany. J. Environ. Manag. 2021, 290, 112557. [Google Scholar] [CrossRef]
- Sara, M.; Rouissi, T.; Brar, S.K.; Blais, J.F. Chapter 4—Life Cycle Analysis of Potential Substrates of Sustainable Biorefinery. In Platform Chemical Biorefinery; Brar, S.K., Sarma, S.J., Pakshirajan, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 55–76. [Google Scholar] [CrossRef]
- Goswami, R.; Thakur, R. Chapter 26—Valorizing sludge: A biorefinery perspective prospecting for sustainable development. In Clean Energy and Resource Recovery; An, A., Tyagi, V., Kumar, M., Cetecioglu, Z., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 435–454. [Google Scholar] [CrossRef]
- Cruce, J.R.; Quinn, J.C. Economic viability of multiple algal biorefining pathways and the impact of public policies. Appl. Energy 2019, 233–234, 735–746. [Google Scholar] [CrossRef]
- Fernando-Foncillas, C.; Varrone, C. Potential of the sewage sludge valorization in Scandinavia by co-digestion with other organic wastes: A techno-economic assessment. J. Clean. Prod. 2021, 324, 129239. [Google Scholar] [CrossRef]
- Houillon, G.; Jolliet, O. Life cycle assessment of processes for the treatment of wastewater urban sludge: Energy and global warming analysis. J. Clean. Prod. 2005, 13, 287–299. [Google Scholar] [CrossRef]
- Kim, H.W.; Han, S.K.; Shin, H.S. The optimisation of food waste addition as a cosubstrate in anaerobic digestion of sewage sludge. Waste Manag. Res. 2003, 21, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Capodaglio, A.G.; Ranieri, E.; Torretta, V. Process enhancement for maximization of methane production in codigestion biogas plants. Manag. Environ. Qual. Int. J. 2016, 27, 289–298. [Google Scholar] [CrossRef]
- Nghiem, L.D.; Koch, K.; Bolzonella, D.; Drewes, J.E. Full scale co-digestion of wastewater sludge and food waste: Bottlenecks and possibilities. Renew. Sustain. Energy Rev. 2017, 72, 354–362. [Google Scholar] [CrossRef] [Green Version]
- Strazzera, G.; Battista, F.; Herrero Garcia, N.; Frison, N.; Bolzonella, D. Volatile fatty acids production from food wastes for biorefinery platforms: A review. J. Environ. Manag. 2018, 226, 278–288. [Google Scholar] [CrossRef]
- Edwards, J.; Burn, S.; Crossin, E.; Othman, M. Life cycle costing of municipal food waste management systems: The effect of environmental externalities and transfer costs using local government case studies. Resour. Conserv. Recycl. 2018, 138, 118–129. [Google Scholar] [CrossRef]




| Parameter | Primary Sludge | Activated Sludge |
|---|---|---|
| Total dry solids (total solids, TS) % | 5–9 | 0.8–1.2 |
| Volatile solids, vs. (%TS) | 60–80 | 59–68 |
| Nitrogen (%TS) | 1.5–4 | 2.4–5.0 |
| Phosphorus (%TS) | 0.8–2.8 | 0.5–0.7 |
| Potash (K2O %TS) | 0–1 | 0.5–0.7 |
| Cellulose (%TS) | 8–15 | 7–9.7 |
| Iron (Fe g/kg) | 2–4 | – |
| Silica (SiO2 %TS) | 15–20 | – |
| pH | 5.0–8.0 | 6.5–8.0 |
| Grease and fats (%TS) | 7–35 | 5–12 |
| Protein (%TS) | 20–30 | 32–41 |
| Alkalinity (mg/L as CaCO3) | 500–1500 | 580–1100 |
| Organic acids (mg/L as acetate) | 200–2000 | 1100–1700 |
| Energy content (kJ/kg TS) | 23,000–29,000 | 19,000–23,000 |
| Biorefinery Concept | Feedstock | TRL * |
|---|---|---|
| Conventional biorefinery | Starch and sugar crops, wood | 9 |
| Whole crop biorefinery | Whole crop (including straw) cereals | 7–8 |
| Oleochemical biorefinery | Oil crops | 7–9 |
| Lignocellulosic biorefinery | Lignocellulosic-rich biomass (wood, straw, etc.) | 6–8 |
| Green biorefineries | Wet biomass: green crops, leaves, food and organic waste | 5–7 |
| Marine biorefineries | Aquatic biomass: micro- and macroalgae | 5–6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capodaglio, A.G. Biorefinery of Sewage Sludge: Overview of Possible Value-Added Products and Applicable Process Technologies. Water 2023, 15, 1195. https://doi.org/10.3390/w15061195
Capodaglio AG. Biorefinery of Sewage Sludge: Overview of Possible Value-Added Products and Applicable Process Technologies. Water. 2023; 15(6):1195. https://doi.org/10.3390/w15061195
Chicago/Turabian StyleCapodaglio, Andrea G. 2023. "Biorefinery of Sewage Sludge: Overview of Possible Value-Added Products and Applicable Process Technologies" Water 15, no. 6: 1195. https://doi.org/10.3390/w15061195