Using Activated Biochar from Caryocar brasiliense Pequi Almonds for Removing Methylene Blue Dye in an Aqueous Solution
Abstract
:1. Introduction
2. Experimental Part
2.1. Reagents
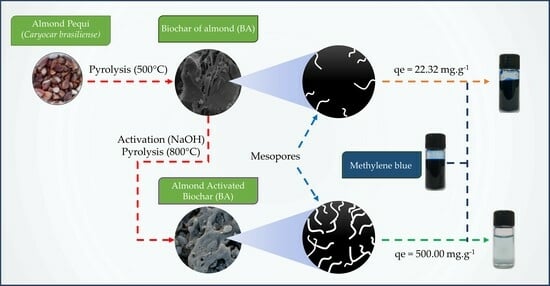
2.2. Biochar Preparation
2.3. Characterizations
2.4. Point of Zero Charge (pHPZC)
2.5. The Influence of pH
2.6. Adsorption Kinetics
2.7. Adsorption Isotherm
3. Results and Discussions
Characterization of Biochars after Adsorption
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmad, R.; Ansari, K. Enhanced Sequestration of Methylene Blue and Crystal Violet Dye onto Green Synthesis of Pectin Modified Hybrid (Pect/AILP-Kal) Nanocomposite. Process Biochem. 2021, 111, 132–143. [Google Scholar] [CrossRef]
- Zaman, K.U.; Abbas, N.; Irshad, M.; Zehra, S.; Butt, M.T.; Shehzad, K.; Mahmood, H.R. Treatability Study of Synthesized Silica Nanoparticles to Reduce Pollution Load of Industrial Wastewater. Int. J. Environ. Sci. Technol. 2022, 19, 6183–6200. [Google Scholar] [CrossRef]
- Lum, P.T.; Foo, K.Y.; Zakaria, N.A.; Palaniandy, P. Ash Based Nanocomposites for Photocatalytic Degradation of Textile Dye Pollutants: A Review. Mater. Chem. Phys. 2020, 241, 122405. [Google Scholar] [CrossRef]
- Nguyen, D.T.C.; Vo, D.V.N.; Nguyen, T.T.; Nguyen, T.T.T.; Nguyen, L.T.T.; Tran, T.V. Kinetic, Equilibrium, Adsorption Mechanisms of Cationic and Anionic Dyes on N-Doped Porous Carbons Produced from Zeolitic-Imidazolate Framework. Int. J. Environ. Sci. Technol. 2022, 19, 10723–10736. [Google Scholar] [CrossRef]
- Jawad, A.H.; Abdulhameed, A.S.; Mastuli, M.S. Mesoporous Crosslinked Chitosan-Activated Charcoal Composite for the Removal of Thionine Cationic Dye: Comprehensive Adsorption and Mechanism Study. J. Polym. Environ. 2020, 28, 1095–1105. [Google Scholar] [CrossRef]
- Oraon, A.; Prajapati, A.K.; Ram, M.; Saxena, V.K.; Dutta, S.; Gupta, A.K. Synthesis, Characterization, and Application of Microporous Biochar Prepared from Pterospermum acerifolium Plant Fruit Shell Waste for Methylene Blue Dye Adsorption: The Role of Surface Modification by SDS Surfactant. Biomass Convers. Biorefin. 2022. [Google Scholar] [CrossRef]
- Prajapati, A.K.; Mondal, M.K. Comprehensive Kinetic and Mass Transfer Modeling for Methylene Blue Dye Adsorption onto CuO Nanoparticles Loaded on Nanoporous Activated Carbon Prepared from Waste Coconut Shell. J. Mol. Liq. 2020, 307, 112949. [Google Scholar] [CrossRef]
- Ali, I.; Basheer, A.A.; Mbianda, X.Y.; Burakov, A.; Galunin, E.; Burakova, I.; Mkrtchyan, E.; Tkachev, A.; Grachev, V. Graphene Based Adsorbents for Remediation of Noxious Pollutants from Wastewater. Environ. Int. 2019, 127, 160–180. [Google Scholar] [CrossRef]
- Mingming, L.; Jing, G.; Piao, Y.; Liu, D.; Jin, L. Treatment of Refractory Organic Pollutants in Industrial Wastewater by Wet Air Oxidation. Arab. J. Chem. 2017, 10, S769–S776. [Google Scholar]
- Hadj-Otmane, C.; Ouakouak, A.; Touahra, F.; Grabi, H.; Martín, J.; Bilal, M. Date Palm Petiole–Derived Biochar: Effect of Pyrolysis Temperature and Adsorption Properties of Hazardous Cationic Dye from Water. Biomass Convers. Biorefin. 2022. [Google Scholar] [CrossRef]
- Reza, M.S.; Afroze, S.; Bakar, M.S.A.; Saidur, R.; Aslfattahi, N.; Taweekun, J.; Azad, A.K. Biochar Characterization of Invasive Pennisetum purpureum Grass: Effect of Pyrolysis Temperature. Biochar 2020, 2, 239–251. [Google Scholar] [CrossRef]
- Hsu, D.; Lu, C.; Pang, T.; Wang, Y.; Wang, G. Adsorption of Ammonium Nitrogen from Aqueous Solution on Chemically Activated Biochar Prepared from Sorghum Distillers Grain. Appl. Sci. 2019, 9, 5249. [Google Scholar] [CrossRef]
- Gümüş, F. Utilization of Algal Waste Biomass-Derived Biochar Prepared by a Microwave-Assisted Method for Aniline Green Adsorption. Water Air Soil Pollut. 2022, 233, 364. [Google Scholar] [CrossRef]
- Vijayaraghavan, K.; Ashokkumar, T. Characterization and Evaluation of Reactive Dye Adsorption onto Biochar Derived from Turbinaria Conoides Biomass. Environ. Prog. Sustain. Energy 2019, 38, 13143. [Google Scholar] [CrossRef]
- Carneiro, M.T.; Barros, A.Z.B.; Morais, A.I.S.; Carvalho Melo, A.L.F.; Bezerra, R.D.S.; Osajima, J.A.; Silva-Filho, E.C. Application of Water Hyacinth Biomass (Eichhornia crassipes) as an Adsorbent for Methylene Blue Dye from Aqueous Medium: Kinetic and Isothermal Study. Polymers 2022, 14, 2732. [Google Scholar] [CrossRef] [PubMed]
- Amorim, D.J.; Rezende, H.C.; Oliveira, É.L.; Almeida, I.L.S.; Coelho, N.M.M.; Matos, T.N.; Araújo, C.S.T. Characterization of Pequi (Caryocar brasiliense) Shells and Evaluation of Their Potential for the Adsorption of Pb II Ions in Aqueous Systems. J. Braz. Chem. Soc. 2016, 27, 616–623. [Google Scholar] [CrossRef]
- Borba, L.L.; Cuba, R.M.F.; Terán, F.J.C.; Castro, M.N.; Mendes, T.A. Use of Adsorbent Biochar from Pequi (Caryocar brasiliense) Husks for the Removal of Commercial Formulation of Glyphosate from Aqueous Media. Braz. Arch. Biol. Technol. 2019, 62, e19180450. [Google Scholar] [CrossRef]
- Melo, A.L.F.C.; Carneiro, M.T.; Nascimento, A.M.S.S.; Morais, A.I.S.; Bezerra, R.D.S.; Viana, B.C.; Osajima, J.A.; Silva-Filho, E.C. Biochar Obtained from Caryocar brasiliense Endocarp for Removal of Dyes from the Aqueous Medium. Materials 2022, 15, 9076. [Google Scholar] [CrossRef]
- Nascimento-Silva, N.R.R.D.; Naves, M.M.V. Potential of Whole Pequi (Caryocar spp.) Fruit-Pulp, Almond, Oil, and Shell-as a Medicinal Food. J. Med. Food 2019, 22, 952–962. [Google Scholar] [CrossRef]
- Ramos, J.P.; Pavão, M.F.U.; Barra, E.C.; Vilhena, K.S.S.; Gouveia, F.P. Potencial de Adsorção Do Resíduo Proveniente Do Processo de Obtenção Do Silício Metálico. Rev. Virtual Quim. 2017, 9, 751–763. [Google Scholar] [CrossRef]
- Santos, D.H.S.; Santos, J.P.T.S.; Duarte, J.L.S.; Oliveira, L.M.T.M.; Tonholo, J.; Meili, L.; Zanta, C.L.P.S. Regeneration of Activated Carbon Adsorbent by Anodic and Cathodic Electrochemical Process. Process Saf. Environ. Prot. 2022, 159, 1150–1163. [Google Scholar] [CrossRef]
- da Silva, L.H.P.; Pinto, L.C.L.; de Melo Teixeira, S.A.; Drumond, M.A. Pequi Fruit (Caryocar brasiliense) in Minas Gerais: Commercialization and Public Policy. Floresta E Ambiente 2020, 27, e20171129. [Google Scholar] [CrossRef]
- Cazetta, A.L.; Vargas, A.M.M.; Nogami, E.M.; Kunita, M.H.; Guilherme, M.R.; Martins, A.C.; Silva, T.L.; Moraes, J.C.G.; Almeida, V.C. NaOH-Activated Carbon of High Surface Area Produced from Coconut Shell: Kinetics and Equilibrium Studies from the Methylene Blue Adsorption. Chem. Eng. J. 2011, 174, 117–125. [Google Scholar] [CrossRef]
- Mu, Y.; Ma, H. NaOH-Modified Mesoporous Biochar Derived from Tea Residue for Methylene Blue and Orange II Removal. Chem. Eng. Res. Des. 2021, 167, 129–140. [Google Scholar] [CrossRef]
- Ferreira, F.J.L.; Silva, L.S.; da Silva, M.S.; Osajima, J.A.; Meneguin, A.B.; Santagneli, S.H.; Barud, H.S.; Bezerra, R.D.S.; Silva-Filho, E.C. Understanding Kinetics and Thermodynamics of the Interactions between Amitriptyline or Eosin Yellow and Aminosilane-Modified Cellulose. Carbohydr. Polym. 2019, 225, 115246. [Google Scholar] [CrossRef]
- Lagergren, S.K. About the Theory of So-Called Adsorption of Soluble Substances. Sven. Vetenskapsakad. Handingarl 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; Mckay, G. Pseudo-Second Order Model for Sorption Processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Über Die Adsorption in Lösungen. Z. Für Phys. Chem. 1907, 57, 385–470. [Google Scholar] [CrossRef]
- Chen, J.; Wang, M.; Wang, S.; Li, X. Hydrogen Production via Steam Reforming of Acetic Acid over Biochar-Supported Nickel Catalysts. Int. J. Hydrog. Energy 2018, 43, 18160–18168. [Google Scholar] [CrossRef]
- Chen, W.; Li, K.; Chen, Z.; Xia, M.; Chen, Y.; Yang, H.; Chen, X.; Chen, H. A New Insight into Chemical Reactions between Biomass and Alkaline Additives during Pyrolysis Process. Proc. Combust. Inst. 2021, 38, 3881–3890. [Google Scholar] [CrossRef]
- Jiang, D.; Li, H.; Cheng, X.; Ling, Q.; Chen, H.; Barati, B.; Yao, Q.; Abomohra, A.; Hu, X.; Bartocci, P.; et al. A Mechanism Study of Methylene Blue Adsorption on Seaweed Biomass Derived Carbon: From Macroscopic to Microscopic Scale. Process Saf. Environ. Prot. 2023, 172, 1132–1143. [Google Scholar] [CrossRef]
- Liu, J.; Cheng, W.; Yang, X.; Bao, Y. Modification of Biochar with Silicon by One-Step Sintering and Understanding of Adsorption Mechanism on Copper Ions. Sci. Total Environ. 2020, 704, 135252. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yang, L.; Wang, C.; Zhang, Q.; Liu, Q.; Li, Y.; Xiao, R. Adsorption of Cd(II) from Aqueous Solutions by Rape Straw Biochar Derived from Different Modification Processes. Chemosphere 2017, 175, 332–340. [Google Scholar] [CrossRef]
- Yan, Q.; Li, J.; Cai, Z. Preparation and Characterization of Chars and Activated Carbons from Wood Wastes. Carbon Lett. 2021, 31, 941–956. [Google Scholar] [CrossRef]
- Zhang, X.; Mao, X.; Pi, L.; Wu, T.; Hu, Y. Adsorptive and Capacitive Properties of the Activated Carbons Derived from Pig Manure Residues. J. Environ. Chem. Eng. 2019, 7, 103066. [Google Scholar] [CrossRef]
- Xu, Z.; He, M.; Xu, X.; Cao, X.; Tsang, D.C.W. Impacts of Different Activation Processes on the Carbon Stability of Biochar for Oxidation Resistance. Bioresour. Technol. 2021, 338, 125555. [Google Scholar] [CrossRef]
- Li, C.; Zhang, X.; Lv, Z.; Wang, K.; Sun, X.; Chen, X.; Ma, Y. Scalable Combustion Synthesis of Graphene-Welded Activated Carbon for High-Performance Supercapacitors. Chem. Eng. J. 2021, 414, 128781. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Chen, C.; Zhu, Z. Low-Cost, High-Performance Supercapacitor Based on Activated Carbon Electrode Materials Derived from Baobab Fruit Shells. J. Colloid. Interface Sci. 2019, 538, 308–319. [Google Scholar] [CrossRef]
- Ren, Z.; Wang, Z.; Lv, L.; Ma, P.; Zhang, G.; Li, Y.; Qin, Y.; Wang, P.; Liu, X.; Gao, W. Fe–N Complex Biochar as a Superior Partner of Sodium Sulfide for Methyl Orange Decolorization by Combination of Adsorption and Reduction. J. Environ. Manag. 2022, 316, 115213. [Google Scholar] [CrossRef]
- Feng, D.; Guo, D.; Zhang, Y.; Sun, S.; Zhao, Y.; Shang, Q.; Sun, H.; Wu, J.; Tan, H. Functionalized Construction of Biochar with Hierarchical Pore Structures and Surface O-/N-Containing Groups for Phenol Adsorption. Chem. Eng. J. 2021, 410, 127707. [Google Scholar] [CrossRef]
- Ye, J.; Tao, S.; Zhao, S.; Li, S.; Chen, S.; Cui, Y. Characteristics of Methane Adsorption/Desorption Heat and Energy with Respect to Coal Rank. J. Nat. Gas. Sci. Eng. 2022, 99, 104445. [Google Scholar] [CrossRef]
- Zhu, H.; Liu, X.; Jiang, Y.; Zhang, M.; Lin, D.; Yang, K. Time-Dependent Desorption of Anilines, Phenols, and Nitrobenzenes from Biochar Produced at 700 °C: Insight into Desorption Hysteresis. Chem. Eng. J. 2021, 422, 130584. [Google Scholar] [CrossRef]
- Eltaweil, A.S.; Ali Mohamed, H.; Abd El-Monaem, E.M.; El-Subruiti, G.M. Mesoporous Magnetic Biochar Composite for Enhanced Adsorption of Malachite Green Dye: Characterization, Adsorption Kinetics, Thermodynamics and Isotherms. Adv. Powder Technol. 2020, 31, 1253–1263. [Google Scholar] [CrossRef]
- Wang, T.; Zheng, J.; Cai, J.; Liu, Q.; Zhang, X. Visible-Light-Driven Photocatalytic Degradation of Dye and Antibiotics by Activated Biochar Composited with K+ Doped g-C3N4: Effects, Mechanisms, Actual Wastewater Treatment and Disinfection. Sci. Total Environ. 2022, 839, 155955. [Google Scholar] [CrossRef]
- Carneiro, M.T.; Morais, A.Í.S.; de Carvalho Melo, A.L.F.; Ferreira, F.J.L.; Santos, F.E.P.; Viana, B.C.; Osajima, J.A.; Bezerra, R.D.S.; Del Mar Orta Cuevas, M.; Peña-Garcia, R.R.; et al. Biochar Derived from Water Hyacinth Biomass Chemically Activated for Dye Removal in Aqueous Solution. Sustainability 2023, 15, 14578. [Google Scholar] [CrossRef]
- Doğan, M.; Sabaz, P.; Bїcїl, Z.; Koçer Kizilduman, B.; Turhan, Y. Activated Carbon Synthesis from Tangerine Peel and Its Use in Hydrogen Storage. J. Energy Inst. 2020, 93, 2176–2185. [Google Scholar] [CrossRef]
- Jawad, A.H.; Abdulhameed, A.S.; Mastuli, M.S. Acid-Factionalized Biomass Material for Methylene Blue Dye Removal: A Comprehensive Adsorption and Mechanism Study. J. Taibah Univ. Sci. 2020, 14, 305–313. [Google Scholar] [CrossRef]
- Suhaimi, N.; Kooh, M.R.R.; Lim, C.M.; Chou Chao, C.-T.; Chou Chau, Y.-F.; Mahadi, A.H.; Chiang, H.-P.; Haji Hassan, N.H.; Thotagamuge, R. The Use of Gigantochloa Bamboo-Derived Biochar for the Removal of Methylene Blue from Aqueous Solution. Adsorpt. Sci. Technol. 2022, 2022, 8245797. [Google Scholar] [CrossRef]
- Kooh, M.R.R.; Thotagamuge, R.; Chou Chau, Y.-F.; Mahadi, A.H.; Lim, C.M. Machine Learning Approaches to Predict Adsorption Capacity of Azolla Pinnata in the Removal of Methylene Blue. J. Taiwan. Inst. Chem. Eng. 2022, 132, 104134. [Google Scholar] [CrossRef]
- Bernal-Romero del Hombre Bueno, M.D.L.Á.; Boluda-Botella, N.; Prats Rico, D. Removal of Emerging Pollutants in Water Treatment Plants: Adsorption of Methyl and Propylparaben onto Powdered Activated Carbon. Adsorption 2019, 25, 983–999. [Google Scholar] [CrossRef]
- Yin, Y.; Zhou, T.; Luo, H.; Geng, J.; Yu, W.; Jiang, Z. Adsorption of Arsenic by Activated Charcoal Coated Zirconium-Manganese Nanocomposite: Performance and Mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2019, 575, 318–328. [Google Scholar] [CrossRef]
- Paschalidou, P.; Pashalidis, I.; Manariotis, I.D.; Karapanagioti, H.K. Hyper Sorption Capacity of Raw and Oxidized Biochars from Various Feedstocks for U(VI). J. Environ. Chem. Eng. 2020, 8, 103932. [Google Scholar] [CrossRef]
- Liu, S.; Shen, C.; Wang, Y.; Huang, Y.; Hu, X.; Li, B.; Karnowo; Zhou, J.; Zhang, S.; Zhang, H. Development of CO2/H2O Activated Biochar Derived from Pine Pyrolysis: Application in Methylene Blue Adsorption. J. Chem. Technol. Biotechnol. 2022, 97, 885–893. [Google Scholar] [CrossRef]
- Qiu, B.; Shao, Q.; Shi, J.; Yang, C.; Chu, H. Application of Biochar for the Adsorption of Organic Pollutants from Wastewater: Modification Strategies, Mechanisms and Challenges. Sep. Purif. Technol. 2022, 300, 121925. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, C.; Huang, Q.; Yan, J. Hierarchical Porous Structure Formation Mechanism in Food Waste Component Derived N-Doped Biochar: Application in VOCs Removal. Chemosphere 2022, 291, 132702. [Google Scholar] [CrossRef]
- Sajjadi, B.; Chen, W.Y.; Egiebor, N.O. A Comprehensive Review on Physical Activation of Biochar for Energy and Environmental Applications. Rev. Chem. Eng. 2019, 35, 735–776. [Google Scholar] [CrossRef]
- Sajjadi, B.; Broome, J.W.; Chen, W.Y.; Mattern, D.L.; Egiebor, N.O.; Hammer, N.; Smith, C.L. Urea Functionalization of Ultrasound-Treated Biochar: A Feasible Strategy for Enhancing Heavy Metal Adsorption Capacity. Ultrason. Sonochem 2019, 51, 20–30. [Google Scholar] [CrossRef]
- Kar, S.; Santra, B.; Kumar, S.; Ghosh, S.; Majumdar, S. Sustainable Conversion of Textile Industry Cotton Waste into P-Dopped Biochar for Removal of Dyes from Textile Effluent and Valorisation of Spent Biochar into Soil Conditioner towards Circular Economy. Environ. Pollut. 2022, 312, 120056. [Google Scholar] [CrossRef]
- dos Santos, K.J.L.; dos Santos, G.E.d.S.; de Sá, Í.M.G.L.; Ide, A.H.; Duarte, J.L.d.S.; de Carvalho, S.H.V.; Soletti, J.I.; Meili, L. Wodyetia bifurcata Biochar for Methylene Blue Removal from Aqueous Matrix. Bioresour. Technol. 2019, 293, 122093. [Google Scholar] [CrossRef]
- Jabar, J.M.; Odusote, Y.A. Utilization of Prepared Activated Biochar from Water Lily (Nymphaea lotus) Stem for Adsorption of Malachite Green Dye from Aqueous Solution. Biomass Convers. Biorefin. 2021. [Google Scholar] [CrossRef]









| Bands | BA Biochar | ABA Biochar | ||||
|---|---|---|---|---|---|---|
| Amplitude | Center | FWHM | Amplitude | Center | FWHM | |
| D1 | 182.48 | 1348.40 | 230.98 | 295.87 | 1305.55 | 162.46 |
| G | 80.82 | 1579.41 | 106.11 | 149.71 | 1576.07 | 68.65 |
| ID1/IG | 2.26 | 1.98 | ||||
| Biochar | Surface Area m2 g−1 | Pore Volume—BJH cm3 g−1 | Average Pore Diameter—BJH (nm) |
|---|---|---|---|
| BA | 14.5 | 0.093 | 3.115 |
| ABA | 1923.0 | 0.290 | 3.133 |
| Biochar | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|
| qe (mg g−1) | K1 (min−1) | R2 | qe (mg g−1) | K2 (mg g−1 min−1) | R2 | |
| BA | 11.20 | 0.0098 | 0.8850 | 22.32 | 0.0022 | 0.9844 |
| ABA | 11.75 | 0.0084 | 0.9453 | 500.00 | 0.0040 | 0.9999 |
| Biochar | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| q0 | KL | R2 | n | KF | R2 | |
| BA | 42.73 | 0.009992 | 0.9268 | 11.0497 | 18.05 | 0.1846 |
| ABA | 476.19 | 0.004671 | 0.9965 | 2.9797 | 41.73 | 0.9643 |
| Bands | BAA Biochar | ABAA Biochar | ||||
|---|---|---|---|---|---|---|
| Amplitude | Center | FWHM | Amplitude | Center | FWHM | |
| D1 | 202.72 | 1336.65 | 244.42 | 122.01 | 1317.40 | 144.73 |
| G | 83.69 | 1569.68 | 97.55 | 57.88 | 1584.28 | 87.26 |
| ID1/IG | 2.42 | 2.11 | ||||
| Biochar | Surface Area m2 g−1 | Pore Volume—BJH cm3 g−1 | Average Pore Diameter—BJH (nm) |
|---|---|---|---|
| BA | 14.5 | 0.093 | 3.115 |
| BAA | 13.4 | 0.027 | 3.180 |
| ABA | 1923.0 | 0.290 | 3.133 |
| ABAA | 598.6 | 0.134 | 3.957 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melo, A.L.F.C.; Carneiro, M.T.; Morais, A.Í.S.; Viana, B.C.; Santos, F.E.P.; Osajima, J.A.; Bezerra, R.D.S.; Peña-Garcia, R.R.; Almeida, L.C.; Carrasco, S.M.; et al. Using Activated Biochar from Caryocar brasiliense Pequi Almonds for Removing Methylene Blue Dye in an Aqueous Solution. Water 2023, 15, 4006. https://doi.org/10.3390/w15224006
Melo ALFC, Carneiro MT, Morais AÍS, Viana BC, Santos FEP, Osajima JA, Bezerra RDS, Peña-Garcia RR, Almeida LC, Carrasco SM, et al. Using Activated Biochar from Caryocar brasiliense Pequi Almonds for Removing Methylene Blue Dye in an Aqueous Solution. Water. 2023; 15(22):4006. https://doi.org/10.3390/w15224006
Chicago/Turabian StyleMelo, André Luiz Ferreira Carvalho, Marcelo Teixeira Carneiro, Alan Ícaro Sousa Morais, Bartolomeu Cruz Viana, Francisco Eroni Paz Santos, Josy Anteveli Osajima, Roosevelt D. S. Bezerra, Ramón Raudel Peña-Garcia, Luciano C. Almeida, Santiago Medina Carrasco, and et al. 2023. "Using Activated Biochar from Caryocar brasiliense Pequi Almonds for Removing Methylene Blue Dye in an Aqueous Solution" Water 15, no. 22: 4006. https://doi.org/10.3390/w15224006