Evaluation of the Potential Release Risk of Internal N and P from Sediments—A Preliminary Study in Two Freshwater Reservoirs in South China
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sediment Sampling and Preparation
2.2. Sediment Analysis
2.3. Sediment N and P Fractionation
2.4. Sediment Incubation and Evaluation of N and P Potential Release Risks
2.5. Statistical Analysis
3. Results
3.1. Sediment Properties and N and P Fractions
3.2. N and P Concentrations and Apparent Fluxes at 15 °C and 25 °C
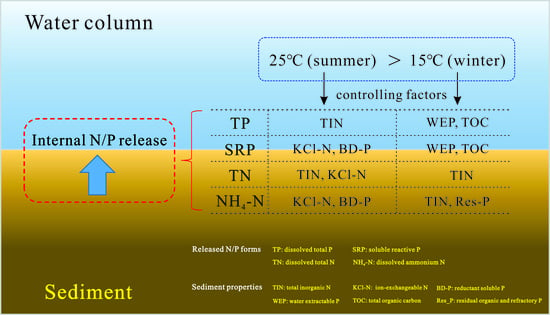
3.3. Controlling Factors of Apparent N and P Fluxes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Conley, D.J.; Paerl, H.W.; Howarth, R.W.; Boesch, D.F.; Seitzinger, S.P.; Havens, K.E. Controlling eutrophication: Nitrogen and phosphorus. Science 2009, 323, 1014–1015. [Google Scholar] [CrossRef] [PubMed]
- Jenny, J.-P.; Normandeau, A.; Francus, P.; Taranu, Z.E.; Gregory-Eaves, I.; Lapointe, F. Urban point sources of nutrients were the leading cause for the historical spread of hypoxia across European lakes. Proc. Natl. Acad. Sci. USA 2016, 113, 12655–12660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Moal, M.; Gascuel-Odoux, C.; Ménesguen, A.; Souchon, Y.; Étrillard, C.; Levain, A. Eutrophication: A new wine in an old bottle? Sci. Total. Environ. 2018, 651, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brooks, B.W.; Lazorchak, J.M.; Howard, M.D.A.; Johnson, M.-V.V.; Morton, S.L.; Perkins, D.A.K.; Steevens, J.A. Are harmful algal blooms becoming the greatest inland water quality threat to public health and aquatic ecosystems? Environ. Toxicol. Chem. 2016, 35, 6–13. [Google Scholar] [CrossRef]
- Ward, M.H.; Jones, R.; Brender, J.; de Kok, T.; Weyer, P.; Nolan, B.; Van Breda, S.G. Drinking water nitrate and human health: An updated review. Int. J. Environ. Res. Public Health 2018, 15, 1557. [Google Scholar] [CrossRef] [Green Version]
- Peñuelas, J.; Poulter, B.; Sardans, J.; Ciais, P.; van der Velde, M.; Bopp, L.; Janssens, I.A. Human-induced nitrogen–phosphorus imbalances alter natural and managed ecosystems across the globe. Nat. Commun. 2013, 2013. 4, 2934. [Google Scholar] [CrossRef] [Green Version]
- Schindler, D.W.; Carpenter, S.R.; Chapra, S.C.; Hecky, R.E.; Orihel, D.M. Reducing phosphorus to curb Lake eutrophication is a success. Environ. Sci. Technol. 2016, 50, 8923–8929. [Google Scholar] [CrossRef]
- Tong, Y.; Zhang, W.; Wang, X.; Couture, R.-M.; Larssen, T.; Zhao, Y.; Lin, Y. Decline in Chinese lake phosphorus concentration accompanied by shift in sources since 2006. Nat. Geosci. 2017, 10, 507–511. [Google Scholar] [CrossRef]
- Matisoff, G.; Kaltenberg, E.M.; Steely, R.L.; Hummel, S.K.; Seo, J.; Gibbons, K.J.; Chaffin, J.D. Internal loading of phosphorus in western Lake Erie. J. Great Lakes Res. 2016, 42, 775–788. [Google Scholar] [CrossRef]
- Schindler, D.W.; Hecky, R.E.; McCullough, G.K. The rapid eutrophication of Lake Winnipeg: Greening under global change. J. Great Lakes Res. 2012, 38, 6–13. [Google Scholar] [CrossRef]
- Schoumans, O.F.; Chardon, W.J.; Bechmann, M.E.; Gascuel-Odoux, C.; Hofman, G.; Kronvang, B. Overview of mitigation options to reduce phosphorus losses from rural areas and to improve surface water quality. Sci. Total Environ. 2014, 468–469, 1255–1266. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, S.R. Eutrophication of aquatic ecosystems: Bistability and soil phosphorus. Proc. Natl. Acad. Sci. USA 2005, 102, 10002–10005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janssen, A.B.G.; de Jager, V.C.L.; Janse, J.H.; Kong, X.; Liu, S.; Ye, Q. Spatial identification of critical nutrient loads of large shallow lakes: Implications for Lake Taihu (China). Water Res. 2017, 119, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Lepori, F.; Roberts, J.J. Effects of internal phosphorus loadings and food-web structure on the recovery of a deep lake from eutrophication. J. Great Lakes Res. 2017, 43, 255–264. [Google Scholar] [CrossRef]
- Watson, S.B.; Miller, C.J.; Arhonditsis, G.B.; Boyer, G.L.; Carmichael, W.W.; Charlton, M.N.; Wilhelm, S.W. The re-eutrophication of Lake Erie: Harmful algal blooms and hypoxia. Harmful Algae 2016, 56, 44–66. [Google Scholar] [CrossRef] [PubMed]
- Cooke, G.D. Internal phosphorus loading in shallow lakes: Importance and control. Lake Reserv. Manag. 2005, 21, 209–217. [Google Scholar] [CrossRef] [Green Version]
- Jeppesen, E.; Søndergaard, M.; Jensen, J.P.; Havens, K.E.; Anneville, O.; Carvalho, L.; Winder, M. Lake responses to reduced nutrient loading—an analysis of contemporary long-term data from 35 case studies. Freshw. Biol. 2005, 50, 1747–1771. [Google Scholar] [CrossRef]
- Ho, J.C.; Michalak, A.M. Phytoplankton blooms in Lake Erie impacted by both longterm and springtime phosphorus loading. J. Great Lakes Res. 2017, 43, 221–228. [Google Scholar] [CrossRef]
- Schindler, D.W.; Hecky, R.E.; Findlay, D.L.; Stainton, M.P.; Parker, B.R.; Paterson, M.J.; Kasian, S. Eutrophication of lakes cannot be controlled by reducing nitrogen input: Results of a 37-year whole-ecosystem experiment. Proc. Natl. Acad. Sci. USA 2008, 105, 11254–11258. [Google Scholar] [CrossRef] [Green Version]
- Lewis, W.M.; Wurtsbaugh, W.A.; Paerl, H.W. Rationale for control of anthropogenic nitrogen and phosphorus to reduce eutrophication of inland waters. Environ. Sci. Technol. 2011, 45, 10300–10305. [Google Scholar] [CrossRef]
- Paerl, H.W.; Scott, J.T.; McCarthy, M.J. It takes two to tango: When and where dual nutrient (N & P) reductions are needed to protect lakes and downstream ecosystems. Environ. Sci. Technol. 2016, 50, 10805–10813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scott, J.T.; McCarthy, M.J. Nitrogen fixation may not balance the nitrogen pool in lakes over timescales relevant to eutrophication management. Limnol. Oceanogr. 2010, 55, 1265–1270. [Google Scholar] [CrossRef]
- Xu, H.; Paerl, H.W.; Qin, B.; Zhu, G.; Hall, N.S.; Wu, Y. Determining critical nutrient thresholds needed to control harmful cyanobacterial blooms in hypertrophic Lake Taihu, China. Environ. Sci. Technol. 2015, 49, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.M.; Chen, M.S.; Gong, M.D.; Fan, X.F.; Qin, B.Q.; Xu, H.; Zhang, C. Internal phosphorus loading from sediments causes seasonal nitrogen limitation for harmful algal blooms. Sci. Total Environ. 2018, 625, 872–884. [Google Scholar] [CrossRef] [PubMed]
- Bullerjahn, G.S.; McKay, R.M.; Davis, T.W.; Baker, D.B.; Boyer, G.L.; D’Anglada, L.V.; Wilhelm, S.W. Global solutions to regional problems: Collecting global expertise to address the problem of harmful cyanobacterial blooms. A Lake Erie case study. Harmful Algae 2016, 54, 223–238. [Google Scholar] [CrossRef] [Green Version]
- Chaffin, J.D.; Bridgeman, T.B.; Bade, D.L. Nitrogen constrains the growth of late summer cyanobacterial blooms in Lake Erie. Adv. Microbiol. 2013, 3, 16. [Google Scholar] [CrossRef] [Green Version]
- Holmroos, H.; Hietanen, S.; Niemisto, J.; Horppila, J. Sediment resuspension and denitrification affect the nitrogen to phosphorus ratio of shallow lake waters. Fundam. Appl. Limnol. 2012, 180, 193–205. [Google Scholar] [CrossRef]
- Yang, C.; Yang, P.; Geng, J.; Yin, H.; Chen, K. Sediment internal nutrient loading in the most polluted area of a shallow eutrophic lake (Lake Chaohu, China) and its contribution to lake eutrophication. Environ. Pollut. 2020, 262, 114292. [Google Scholar] [CrossRef]
- Ma, S.N.; Wang, H.J.; Wang, H.Z.; Li, Y.; Liu, M.; Liang, X.M.; Søndergaard, M. High ammonium loading can increase alkaline phos- phatase activity and promote sediment phosphorus release: A two-month meso- cosm experiment. Water Res. 2018, 145, 388–397. [Google Scholar] [CrossRef]
- Ma, S.N.; Wang, H.J.; Wang, H.Z.; Zhang, M.; Li, Y.; Bian, S.J.; Jeppesen, E. Effects of nitrate on phosphorus release from lake sediments. Water Res. 2021, 194, 116894. [Google Scholar] [CrossRef]
- Chalar, G.; Tundisi, J.G. Phosphorus fractions and fluxes in the water column and sediments of a tropical reservoir (Lobo-Broa-SP). Int. Rev. Hydrobiol. 2015, 86, 183–194. [Google Scholar] [CrossRef]
- Rydin, E. Potentially mobile phosphorus in Lake Erken sediment. Water Res. 2000, 34, 2037–2042. [Google Scholar] [CrossRef]
- Wang, W.W.; Jiang, X.; Zheng, B.H.; Chen, J.Y.; Zhao, L.; Zhang, B.; Wang, S.H. Composition, mineralization potential and release risk of nitrogen in the sediments of Keluke Lake, a Tibetan Plateau freshwater lake in China. R. Soc. Open Sci. 2018, 5, 180612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markovic, S.; Liang, A.; Watson, S.B.; Guo, J.; Mugalingam, S.; Arhonditsis, G.; Dittrich, M. Biogeochemical mechanisms controlling phosphorus diagenesis and internal loading in a remediated hard water eutrophic embayment. Chem. Geol. 2019, 514, 122–137. [Google Scholar] [CrossRef]
- Porubsky, W.P.; Weston, N.B.; Joye, S.B. Benthic metabolism and the fate of dissolved inorganic nitrogen in intertidal sediments. Estuar. Coast. Shelf Sci. 2009, 83, 392–402. [Google Scholar] [CrossRef]
- Burger, D.F.; Hamilton, D.P.; Pilditch, C.A.; Gibbs, M.M. Benthic nutrient fluxes in a eutrophic, polymictic lake. Hydrobiologia 2007, 584, 13–25. [Google Scholar] [CrossRef]
- Jiang, X.; Jin, X.; Yao, Y.; Li, L.; Wu, F. Effects of biological activity, light, temperature and oxygen on phosphorus release processes at the sediment and water interface of Taihu Lake, China. Water Res. 2008, 42, 2251–2259. [Google Scholar] [CrossRef] [PubMed]
- Liikanen, A.; Murtoniemi, T.; Tanskanen, H.; Vaisanen, T.; Martikainen, P.J. Effects of temperature and oxygen availability on greenhouse gas and nutrient dynamics in sediment of a eutrophic mid-boreal lake. Biogeochemistry 2002, 59, 269–286. [Google Scholar] [CrossRef]
- Müller, S.; Mitrovic, S.M.; Baldwin, D.S. Oxygen and dissolved organic carbon control release of N, P and Fe from the sediments of a shallow, polymictic lake. J. Soil. Sediment. 2016, 16, 1109–1120. [Google Scholar] [CrossRef]
- Jackson, M. Soil Chemical Analysis; Prentice Hall of India Pvt. Ltd.: New Delhi, India, 1973; p. 498. [Google Scholar]
- Anderson, G. Factors affecting the estimation of phosphate-ester in soils. J. Sci. Food Agr. 1960, 11, 497–503. [Google Scholar] [CrossRef]
- Nelson, D.W.; Sommers, L.E. Total nitrogen analysis of soil and plant tissues. J. AOAC Int. 1980, 63, 770–778. [Google Scholar] [CrossRef]
- Li, Q.M.; Wang, X.X.; Bartlett, R.; Pinay, G.; Kan, D.; Zhang, W.; Sun, J. Ferrous iron phosphorus in sediments: Development of a quantification method through 2,2′-bipyridine extraction. Water Environ. Res. 2012, 84, 2037–2044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hupfer, M.; Gachter, R.; Giovanoli, R. Transformation of phosphorus species in settling seston and during early sediment diagenesis. Aquat. Sci. 1995, 57, 305–324. [Google Scholar] [CrossRef]
- Huo, S.; Zhang, J.; Xi, B.; Zan, F.Y.; Su, J.; Yu, H. Distribution of nitrogen forms in surface sediments of lakes from different regions, China. Environ. Earth Sci. 2014, 71, 2167–2175. [Google Scholar] [CrossRef]
- Jiao, Y.; Xu, L.; Li, Q.; Gu, S. Thin-layer fine-sand capping of polluted sediments decreases nutrients in overlying water of Wuhan Donghu Lake in China. Environ. Sci. Pollut. Res. 2020, 27, 7156–7165. [Google Scholar] [CrossRef]
- Wang, S.; Qian, X.; Han, B.P.; Wang, Q.H.; Ding, Z.F. Physical limnology of a typical subtropical reservoir in south China. Lake Reserv. Manag. 2011, 27, 149–161. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, F.A.J. Determination of Nitrate in Water Ultraviolet Spectrophotometry. Anal. Chem. 1963, 35, 1292–1294. [Google Scholar] [CrossRef]
- James, G.; Witten, D.; Hastie, T.; Tibshirani, R. An Introduction to Statistical Learning with Applications in R; Springer: New York, NY, USA, 2013; pp. 101–102. [Google Scholar]
- R Core Team. R: A language and environment for statistical computing. In R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2019. [Google Scholar]
- Søndergaard, M.; Jensen, J.P.; Jeppesen, E. Role of sediment and internal loading of phosphorus in shallow lakes. Hydrobiologia 2003, 506, 135–145. [Google Scholar] [CrossRef]
- Gu, S.; Gruau, G.; Dupas, R.; Petitjean, P.; Li, Q.; Pinay, G. Respective roles of Fe-oxyhydroxide dissolution, pH changes and sediment inputs in dissolved phosphorus release from wetland soils under anoxic conditions. Geoderma 2019, 338, 365–374. [Google Scholar] [CrossRef]
- Hicks, N.; Liu, X.; Gregory, R.; Kenny, J.; Lucaci, A.; Lenzi, L.; Duncan, K.R. Temperature driven changes in benthic bacterial diversity influences biogeochemical cycling in coastal sediments. Front. Microbiol. 2018, 9, 1730. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Chen, X.; Yang, X. Effect of organic matter on phosphorus adsorption and desorption in a black soil from Northeast China. Soil Tillage Res. 2019, 187, 85–91. [Google Scholar] [CrossRef]







| Parameter | H1 | H2 | N1 | N2 |
|---|---|---|---|---|
| pH | 5.81 ± 0.22 b | 5.72 ± 0.09 b | 6.25 ± 0.15 a | 6.28 ± 0.14 a |
| Eh | −50.80 ± 31.92 b | −14.25 ± 20.56 b | −252.40 ± 117.16 a | −167.25 ± 91.63 a |
| TOC g kg−1 | 31.97 ± 11.11 bc | 24.04 ± 13.86 c | 59.81 ± 3.33 a | 39.14 ± 18.32 b |
| TNS g kg−1 | 1.45 ± 0.55 b | 1.14 ± 0.95 b | 3.60 ± 1.01 a | 1.95 ± 0.90 b |
| TPS g kg−1 | 0.24 ± 0.06 c | 0.10 ± 0.04 d | 0.71 ± 0.08 a | 0.49 ± 0.21 b |
| OP g kg−1 | 0.13 ± 0.06 c | 0.05 ± 0.02 c | 0.32 ± 0.12 a | 0.23 ± 0.12 b |
| WEP mg kg−1 | 1.30 ± 0.37 b | 1.62 ± 1.17 ab | 2.01 ± 1.73 ab | 2.72 ± 2.05 a |
| TFe g kg−1 | 27.35 ± 14.79 a | 15.74 ± 8.85 b | 22.38 ± 14.34 ab | 24.92 ± 13.81 ab |
| Parameter | H1 | H2 | N1 | N2 |
|---|---|---|---|---|
| NH4Cl-P | 0.24 ± 0.21 b | 0.87 ± 0.48 a | 0.54 ± 0.10 b | 0.77 ± 0.21 a |
| BD-P | 23.48 ± 9.25 c | 19.26 ± 10.39 c | 92.29 ± 10.65 b | 167.92 ± 11.19 a |
| NaOH-P | 82.56 ± 27.14 b | 37.78 ± 19.46 c | 199.76 ± 22.36 a | 184.39 ± 23.20 a |
| HCl-P | 1.32 ± 0.51 b | 0.52 ± 0.50 b | 9.04 ± 2.39 a | 10.21 ± 4.36 a |
| Res-P | 53.47 ± 19.23 c | 23.72 ± 17.45 c | 147.84 ± 35.33 a | 77.06 ± 32.23 b |
| KCl-N | 0.09 ± 0.03 b | 0.07 ± 0.02 b | 0.22 ± 0.05 a | 0.07 ± 0.03 b |
| H2SO4-N | 0.02 ± 0.01 b | 0.02 ± 0.01 b | 0.05 ± 0.01 a | 0.03 ± 0.01 b |
| Org-N | 1.34 ± 0.55 b | 1.05 ± 0.95 b | 3.33 ± 1.01 a | 1.86 ± 0.90 ab |
| Dependent Variables | Explanatory Equation | Adjusted R2 | p-Value |
|---|---|---|---|
| Hongchaojiang (HCJ) | |||
| Flux_15 °C_TP | −1.54[TOC] + 54.32 | 0.19 | 0.135 |
| Flux_15 °C_SRP | –1.86[TOC] + 85.74 | 0.62 | 0.007 |
| Flux_25 °C_TP | –0.83[TIN] + 230.30 | 0.39 | 0.04 |
| Flux_25 °C_SRP | 0.89[BD-P] − 6.23[WEP] + 104.66 | 0.70 | 0.012 |
| Flux_15 °C_TN | 0.40[TIN] − 32.79 | 0.75 | <0.002 |
| Flux_15 °C_NH4_N | 0.10[TIN] − 6.38 | 0.87 | <0.001 |
| Flux_25 °C_TN | 0.36[KCl-N] + 0.26[NaOH-P] − 11.55 | 0.69 | 0.012 |
| Flux_25 °C_NH4_N | 0.06[KCl-N] + 0.05[BD-P] − 3.56 | 0.94 | <0.001 |
| Niuweiling (NWL) | |||
| Flux_15 °C_TP | 36.73[WEP] − 50.80 | 0.40 | 0.040 |
| Flux_15 °C_SRP | 12.31[WEP] + 5.34 | 0.39 | 0.042 |
| Flux_25 °C_TP | −1.48[TIN] + 290.20 | 0.78 | 0.001 |
| Flux_25 °C_SRP | −3.25[KCl-N] + 2.52[TIN] + 110.11 | 0.61 | 0.025 |
| Flux_15 °C_TN | 0.11[TIN] − 0.39 | 0.84 | <0.001 |
| Flux_15 °C_NH4_N | −0.01[Res-P] + 0.75 | 0.00 | 0.51 |
| Flux_25 °C_TN | 0.14[TIN] − 0.35[BD-P] + 60.37 | 0.82 | 0.002 |
| Flux_25 °C_NH4_N | −0.24[BD-P] + 0.48[TNS] + 4.26 | 0.82 | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, P.; Bao, X.; Jiao, Y.; Zhang, X.; Li, Q.; Gu, S. Evaluation of the Potential Release Risk of Internal N and P from Sediments—A Preliminary Study in Two Freshwater Reservoirs in South China. Water 2022, 14, 664. https://doi.org/10.3390/w14040664
Cheng P, Bao X, Jiao Y, Zhang X, Li Q, Gu S. Evaluation of the Potential Release Risk of Internal N and P from Sediments—A Preliminary Study in Two Freshwater Reservoirs in South China. Water. 2022; 14(4):664. https://doi.org/10.3390/w14040664
Chicago/Turabian StyleCheng, Peng, Xu Bao, Yang Jiao, Xuezhi Zhang, Qingman Li, and Sen Gu. 2022. "Evaluation of the Potential Release Risk of Internal N and P from Sediments—A Preliminary Study in Two Freshwater Reservoirs in South China" Water 14, no. 4: 664. https://doi.org/10.3390/w14040664