The Partial Contribution of Constructed Wetland Components (Roots, Gravel, Microorganisms) in the Removal of Phenols: A Mini Review
Abstract
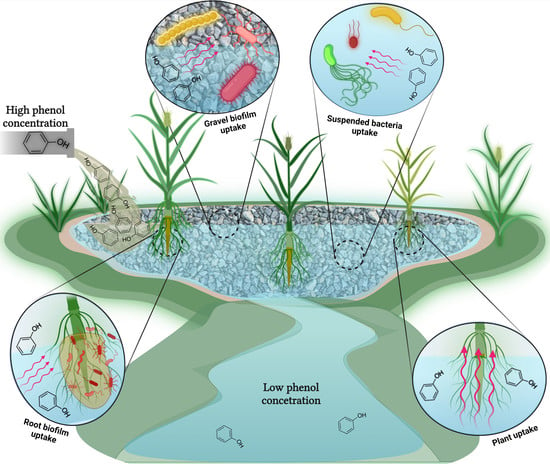
:1. Introduction
2. The Partial Contribution of Plants to Phenol Removal
3. The Partial Contribution of Planktonic Bacteria and Gravel/Root Biofilms to Phenol Removal
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kadlec, R.; Knight, R. Treatment Wetlands; Lewis Publishers: Chelsea, MI, USA, 1996. [Google Scholar]
- Vymazal, J. (Ed.) Natural and Constructed Wetlands: Nutrients, Heavy Metals and Energy Cycling, and Flow; Springer: Cham, Switzerland, 2016. [Google Scholar]
- Vymazal, J. Horizontal sub-surface flow and hybrid constructed wetlands systems for wastewater treatment. Ecol. Eng. 2005, 25, 478–490. [Google Scholar] [CrossRef]
- Ji, Z.; Tang, W.; Pei, Y. Constructed wetland substrates: A review on development, function mechanisms, and application in contaminants removal. Chemosphere 2022, 286, 131564. [Google Scholar] [CrossRef]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef] [PubMed]
- Otte, M.L.; Jacob, D.L. Constructed wetlands for phytoremediation: Rhizofiltration, phytostabilisation and phytoextraction. In Phytoremediation and Rizhoremediation; Mackova, M., Dowling, D.N., Macek, T., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 57–67. [Google Scholar]
- Danhorn, T.; Fuqua, C. Biofilm formation by plant-associated bacteria. Annu. Rev. Microbiol. 2007, 61, 401–422. [Google Scholar] [CrossRef]
- Stottmeister, U.; Wießner, A.; Kuschk, P.; Kappelmeyer, U.; Kästner, M.; Bederski, O.; Müller, R.; Moormann, H. Effects of plants and microorganisms in constructed wetlands for wastewater treatment. Biotechnol. Adv. 2003, 22, 93–117. [Google Scholar] [CrossRef]
- Brix, H. Functions of macrophytes in constructed wetlands. Water Sci. Technol. 1994, 29, 71–78. [Google Scholar] [CrossRef]
- Vacca, G.; Wand, H.; Nikolausz, M.; Kuschk, P.; Kästner, M. Effect of plants and filter materials on bacteria removal in pilot-scale constructed wetlands. Water Res. 2005, 39, 1361–1373. [Google Scholar] [CrossRef]
- Zhu, H.; Zhou, Q.-W.; Yan, B.-X.; Liang, Y.-X.; Yu, X.-F.; Gerchman, Y.; Cheng, X.-W. Influence of vegetation type and temperature on the performance of constructed wetlands for nutrient removal. Water Sci. Technol. 2018, 77, 829–837. [Google Scholar] [CrossRef]
- Zhao, C.; Xu, J.; Shang, D.; Zhang, Y.; Zhang, J.; Xie, H.; Kong, Q.; Wang, Q. Application of constructed wetlands in the PAH remediation of surface water: A review. Sci. Total Environ. 2021, 780, 146605. [Google Scholar] [CrossRef]
- Nzila, A. Biodegradation of high-molecular-weight polycyclic aromatic hydrocarbons under anaerobic conditions: Overview of studies, proposed pathways and future perspectives. Environ. Pollut. 2018, 239, 788–802. [Google Scholar] [CrossRef]
- Srivastava, S.; Kumar, M. Biodegradation of polycyclic aromatic hydrocarbons (PAHs): A sustainable approach. In Sustainable Green Technologies for Environmental Management; Shah, S., Venkatramaman, V., Prasad, R., Eds.; Springer: Singapore, 2019; pp. 111–139. [Google Scholar]
- Seidel, K. Phenol-Abbau in Wasser durch Scirpus lacustris L. wehrend einer versuchsdauer von 31 Monaten. Naturwissenschaften 1965, 52, 398–406. [Google Scholar] [CrossRef]
- Polprasert, C.; Dan, N.P.; Thayalakumaran, N. Application of constructed wetlands to treat some toxic wastewaters under tropical conditions. Water Sci. Technol. 1996, 34, 165–171. [Google Scholar] [CrossRef]
- Abira, M.A.; van Bruggen, J.J.A.; Denny, P. Potential of a tropical subsurface constructed wetland to remove phenol from pre-treated pulp and papermill wastewater. Water Sci. Technol. 2005, 51, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Tee, H.C.; Seng, C.E.; Noor, A.M.; Lim, P.E. Performance comparison of constructed wetlands with gravel- and rice husk-based media for phenol and nitrogen removal. Sci. Total Environ. 2009, 407, 3563–3571. [Google Scholar] [CrossRef]
- Poerschmann, J.; Schultze-Nobre, L. Sorption determination of phenols and polycyclic aromatic hydrocarbons in a multiphase constructed wetland system by solid phase microextraction. Sci. Total Environ. 2014, 482, 234–240. [Google Scholar] [CrossRef]
- Abedi, T.; Mojiri, A. Constructed wetland modified by biochar/zeolite addition for enhanced wastewater treatment. Environ. Technol. Innov. 2019, 16, 100472. [Google Scholar] [CrossRef]
- Stefanakis, A.I.; Thullner, M. Fate of phenolic compounds in constructed wetlands treating contaminated water. In Phytoremediation; Ansari, A.A., Gill, S.S., Gill, R., Lanza, G.R., Newman, L., Eds.; Springer: Cham, Switzerland, 2016; pp. 311–325. [Google Scholar]
- Mojiri, A.; Ahmad, Z.; Tajuddin, R.M.; Arshad, M.F.; Gholami, A. Ammonia, phosphate, phenol, and copper(II) removal from aqueous solution by subsurface and surface flow constructed wetland. Environ. Monit. Assess. 2017, 189, 337. [Google Scholar] [CrossRef]
- Kurzbaum, E.; Zimmels, Y.; Kirzhner, F.; Armon, R. Removal of phenol in a constructed wetland system and the relative contribution of plant roots, microbial activity and porous bed. Water Sci. Technol. 2010, 62, 1327–1334. [Google Scholar] [CrossRef]
- Cunningham, S.D.; Anderson, T.A.; Schwab, A.P.; Hsu, F.C. Phytoremediation of soils contaminated with organic pollutants of outstanding interest. Adv. Agron. 1996, 56, 55–114. [Google Scholar]
- McFarlane, J.C.; Pfleeger, T.; Fletcher, J. Transpiration effect on the uptake and distribution of bromacil, nitrobenzene, and phenol in soybean plants. J. Environ. Qual. 1987, 16, 372–376. [Google Scholar] [CrossRef]
- Flocco, C.G.; Lo Balbo, A.; Carranza, M.P.; Giulietti, A.M. Removal of phenol by alfalfa plants (Medicago sativa L.) grown in hydroponics and its effect on some physiological parameters. Acta Biotechnol. 2002, 22, 43–54. [Google Scholar] [CrossRef]
- Ucisik, A.S.; Trapp, S. Uptake, removal, accumulation, and phytotoxicity of phenol in willow trees (Salix viminalis). Environ. Toxicol. Chem. 2006, 25, 2455–2460. [Google Scholar] [CrossRef] [PubMed]
- Kurzbaum, E.; Kirzhner, F.; Armon, R. A Hydroponic system for growing gnotobiotic vs. sterile plants to study phytoremediation processes. Int. J. Phytoremediat. 2014, 16, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Gersberg, R.M.; Eikins, B.V.; Lyon, S.R.; Goldman, C.R. Role of aquatic plants in wastewater treatment by artificial wetlands. Water Res. 1986, 20, 363–368. [Google Scholar] [CrossRef]
- Brix, H. Do macrophytes play a role in constructed treatment wetland? Water Sci. Technol. 1997, 35, 7–11. [Google Scholar] [CrossRef]
- Tanner, C.C. Plants for constructed wetland treatment systems—A comparison of the growth and nutrient uptake characteristics of eight emergent species. Ecol. Eng. 1996, 7, 59–83. [Google Scholar] [CrossRef]
- Brisson, J.; Chazarenc, F. Maximizing pollutant removal in constructed wetlands: Should we pay more attention to macrophyte species selection? Sci. Total Environ. 2009, 407, 3923–3930. [Google Scholar] [CrossRef]
- Zimmels, Y.; Kirzhner, F.; Schreiber, J. Removal of high organic loads from winery wastewater by aquatic plants. Water Environ. Res. 2008, 80, 806–822. [Google Scholar] [CrossRef]
- Tsao, D.T. Phytoremediation: Advances in Biochemical Engineering/Biotechnology; Springer: Berlin/Heidelberg, Germany, 2003; Volume 78. [Google Scholar]
- Kurzbaum, E.; Kirzhner, F.; Sela, S.; Zimmels, Y.; Armon, R. Efficiency of phenol biodegradation by planktonic Pseudomonas pseudoalcaligenes (a constructed wetland isolate) vs. root and gravel biofilm. Water Res. 2010, 44, 5021–5031. [Google Scholar] [CrossRef]
- Shelef, O.; Gross, A.; Rachmilevitch, S. Role of plants in a constructed wetland: Current and new perspectives. Water 2013, 5, 405–419. [Google Scholar] [CrossRef]
- Sikkema, J.; de Bont, J.A.; Poolman, B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 1995, 59, 201–222. [Google Scholar] [CrossRef] [PubMed]
- Krastanov, A.; Alexieva, Z.; Yemendzhiev, H. Microbial degradation of phenol and phenolic derivatives. Eng. Life Sci. 2013, 13, 76–87. [Google Scholar] [CrossRef]
- Gagnon, V.; Chazarenc, F.; Comeau, Y.; Brisson, J. Influence of macrophyte species on microbial density and activity in constructed wetlands. Water Sci. Technol. 2007, 56, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Pollard, P.C.; Flood, J.A.; Ashbolt, N.J. The direct measurement of bacterial growth in biofilms of emergent plants (Schoenoplectus) of an artificial wetland. Water Sci. Technol. 1995, 32, 251–256. [Google Scholar] [CrossRef]
- Toyama, T.; Yu, N.; Kumada, H.; Sei, K.; Ike, M.; Fujita, M. Accelerated aromatic compounds degradation in aquatic environment by use of interaction between Spirodela polyrrhiza and bacteria in its rhizosphere. J. Biosci. Bioeng. 2006, 101, 346–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, B.; McArthur, J.V.; Sharitz, R.R. Plant effects on microbial assemblages and remediation of acidic coal pile runoff in mesocosm treatment wetlands. Ecol. Eng. 2004, 23, 107–115. [Google Scholar] [CrossRef]
- Polprasert, C.; Khatiwada, N.R. An integrated kinetic model for water hyacinth ponds used for wastewater treatment. Water Res. 1998, 32, 179–185. [Google Scholar] [CrossRef]
- Van Loosdrecht, M.C.M.; Lyklema, J.; Norde, W.; Zehnder, A.J.B. Influence of interfaces on microbial activity. Microbiol. Rev. 1990, 54, 75–87. [Google Scholar] [CrossRef]
- Dawson, M.P.; Humhrey, B.A.; Marshall, K.C. Adhesion: A tactic in the survival strategy of a marine vibrio during starvation. Curr. Microbiol. 1981, 6, 195–198. [Google Scholar] [CrossRef]
- Tanner, C. Plants as ecosystem engineers in subsurface-flow treatment wetlands. Water Sci. Technol. 2001, 44, 9–17. [Google Scholar] [CrossRef]
- Kurzbaum, E.; Kirzhner, F.; Armon, R. Performance comparison of plant root biofilm, gravel attached biofilm and planktonic microbial populations, in phenol removal within a constructed wetland wastewater treatment system. Water SA 2016, 42, 166–170. [Google Scholar] [CrossRef] [Green Version]
- Moormann, H.; Kuschk, P.; Stottmeister, U. The effect of rhizodeposition from helophytes on bacterial degradation of phenolic compounds. Acta Biotechnol. 2002, 22, 107–112. [Google Scholar] [CrossRef]
- Varma, A.; Abbott, L.; Werner, D.; Hampp, R. Plant Surface Microbiology; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2007. [Google Scholar]
- Gilbert, E.S.; Crowley, D. Plant compounds that induce polychlorinated biphenyl biodegradation by Arthrobacter sp. strain B1B. Appl. Environ. Microbiol. 1997, 63, 1933–1938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casavant, N.C.; Thompson, D.; Beattie, G.A.; Phillips, G.J.; Halverson, L.J. Use of a site-specific recombination-based biosensor for detecting bioavailable toluene and related compounds on roots. Environ. Microbiol. 2003, 5, 238–249. [Google Scholar] [CrossRef]
- Rentz, J.A.; Alvarez, P.J.; Schnoor, J.L. Benzo[a]pyrene co-metabolism in the presence of plant root extracts and exudates: Implications for phytoremediation. Environ. Pollut. 2005, 136, 477–484. [Google Scholar] [CrossRef]
- Hussain, F.; Mustufa, G.; Zia, R.; Faiq, A.; Matloob, M.; Shah, H.-U.-R.; Ali, W.R.; Irfan, J.A. Constructed wetlands and their role in remediation of industrial effluents via plant-microbe interaction—A mini review. J. Bioremediat. Biodegrad. 2018, 9, 447. [Google Scholar] [CrossRef]
- Barton, A.J.; Sagers, R.D.; Pitt, W.G. Measurement of bacterial growth rates on polymers. J. Biomed. Mater. Res. 1996, 32, 271–278. [Google Scholar] [CrossRef]
- Heffernan, B.; Murphy, C.D.; Casey, E. Comparison of planktonic and biofilm cultures of Pseudomonas fluorescens DSM 8341 cells grown on fluoroacetate. Appl. Environ. Microbiol. 2009, 75, 2899–2907. [Google Scholar] [CrossRef] [Green Version]
- Jeffrey, W.H.; Paul, J.H. Activity of an attached and free-living Vibrio sp. as measured by thymidine incorporation, p-iodonitrotetrazolium reduction, and ATP/ADP ratios. Appl. Environ. Microbiol. 1986, 51, 150–156. [Google Scholar] [CrossRef] [Green Version]
- Ascon-Cabrera, M.A.; Ascon-Reyes, D.B.; Lebeault, J.M. Degradation activity of adhered and suspended Pseudomonas cells cultured on 2,4,6-trichlorophenol, measured by indirect conductimetry. J. Appl. Bacteriol. 1995, 79, 617–624. [Google Scholar] [CrossRef]
- Bester, E.; Wolfaardt, G.; Joubert, L.; Garny, K.; Saftic, S. Planktonic-cell yield of a pseudomonad biofilm. Appl. Environ. Microbiol. 2005, 71, 7792–7798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bright, J.J.; Fletcher, M. Amino acid assimilation and electron transport system activity in attached and free-living marine bacteria. Appl. Environ. Microbiol. 1983, 45, 818–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iriberri, J.; Unanue, M.; Barcina, I.; Egea, L. Bacterial production and growth rate estimation from [3 H]thymidine incorporation for attached and free-living bacteria in aquatic systems. Appl. Environ. Microbiol. 1990, 56, 483–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurzbaum, E. The Partial Contribution of Constructed Wetland Components (Roots, Gravel, Microorganisms) in the Removal of Phenols: A Mini Review. Water 2022, 14, 626. https://doi.org/10.3390/w14040626
Kurzbaum E. The Partial Contribution of Constructed Wetland Components (Roots, Gravel, Microorganisms) in the Removal of Phenols: A Mini Review. Water. 2022; 14(4):626. https://doi.org/10.3390/w14040626
Chicago/Turabian StyleKurzbaum, Eyal. 2022. "The Partial Contribution of Constructed Wetland Components (Roots, Gravel, Microorganisms) in the Removal of Phenols: A Mini Review" Water 14, no. 4: 626. https://doi.org/10.3390/w14040626