Adsorption of Arsenic on Fe-Modified Biochar and Monitoring Using Spectral Induced Polarization
Abstract
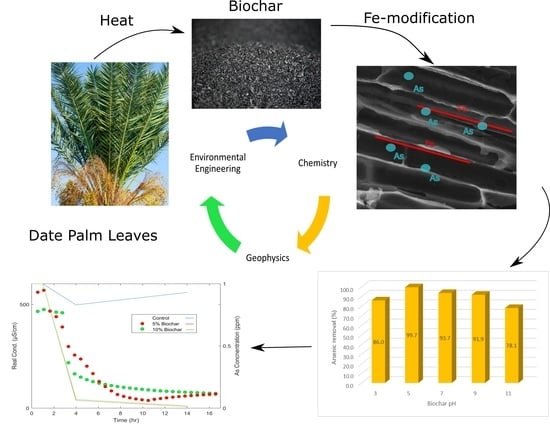
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Batch Adsorption Studies
2.2.2. SIP Monitoring
3. Results and Discussion
3.1. Biochar Characterization
3.2. Adsorption Study
3.3. SIP Monitoring
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ouda, O. Domestic water demand in Saudi Arabia: Assessment of desalinated water as strategic supply source. Desalin. Water Treat. 2014, 56, 2824–2834. [Google Scholar] [CrossRef]
- DeNicola, E.; Aburizaiza, O.S.; Siddique, A.; Khwaja, H.; Carpenter, D.O. Climate Change and Water Scarcity: The Case of Saudi Arabia. Ann. Glob. Health 2015, 81, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Siddiq, M.; Tawabini, B.; Kirmizakis, P.; Kalderis, D.; Ntarlagiannis, D.; Soupios, P. Combining geophysics and material science for environmental remediation: Real-time monitoring of Fe-biochar arsenic wastewater treatment. Chemosphere 2021, 284, 131390. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Arsenic in Drinking-Water. In Guidelines for Drinking-Water Quality; WHO Press: Geneva, Switzerland, 2011; ISBN 9789241548151. [Google Scholar]
- Merola, R.B.; Hien, T.T.; Quyen, D.T.T.; Vengosh, A. Arsenic exposure to drinking water in the Mekong Delta. Sci. Total Environ. 2015, 511, 544–552. [Google Scholar] [CrossRef]
- Rodríguez-Lado, L.; Sun, G.; Berg, M.; Zhang, Q.; Xue, H.; Zheng, Q.; Johnson, C.A. Groundwater Arsenic Contamination Throughout China. Science 2013, 341, 866–868. [Google Scholar] [CrossRef]
- Alidokht, L.; Anastopoulos, I.; Ntarlagiannis, D.; Soupios, P.; Tawabini, B.; Kalderis, D.; Khataee, A. Recent advances in the application of nanomaterials for the remediation of arsenic-contaminated water and soil. J. Environ. Chem. Eng. 2021, 9, 105533. [Google Scholar] [CrossRef]
- Reeder, R.J.; Schoonen, M.A.A.; Lanzirotti, A. Metal Speciation and Its Role in Bioaccessibility and Bioavailability. Rev. Miner. Geochem. 2006, 64, 59–113. [Google Scholar] [CrossRef]
- Pothier, M.P.; Hinz, A.J.; Poulain, A.J. Insights Into Arsenite and Arsenate Uptake Pathways Using a Whole Cell Biosensor. Front. Microbiol. 2018, 9, 2310. [Google Scholar] [CrossRef]
- Nicomel, N.R.; Leus, K.; Folens, K.; Van Der Voort, P.; Du Laing, G. Technologies for Arsenic Removal from Water: Current Status and Future Perspectives. Int. J. Environ. Res. Public Health 2015, 13, 62. [Google Scholar] [CrossRef]
- Mudzielwana, R.; Gitari, M.W.; Ndungu, P. Performance evaluation of surfactant modified kaolin clay in As(III) and As(V) adsorption from groundwater: Adsorption kinetics, isotherms and thermodynamics. Heliyon 2019, 5, e02756. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, A.; Paul, B. The global menace of arsenic and its conventional remediation—A critical review. Chemosphere 2016, 158, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Oehmen, A.; Valerio, R.; Llanos, J.; Fradinho, J.; Serra, S.; Reis, M.A.M.; Crespo, J.G.; Velizarov, S. Arsenic removal from drinking water through a hybrid ion exchange membrane—Coagulation process. Sep. Purif. Technol. 2011, 83, 137–143. [Google Scholar] [CrossRef]
- Pakzadeh, B.; Batista, J.R. Surface complexation modeling of the removal of arsenic from ion-exchange waste brines with ferric chloride. J. Hazard. Mater. 2011, 188, 399–407. [Google Scholar] [CrossRef]
- Navarathna, C.; Alchouron, J.; Liyanage, A.; Herath, A.; Wathudura, P.; Nawalage, S.; Rodrigo, P.; Gunatilake, S.; Mohan, D.; Pittman, C.; et al. Recent Developments in Aqueous Arsenic(III) Remediation Using Biomass-Based Adsorbents. In Contaminants in Our Water: Identification and Remediation Methods; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2020; Volume 1352, pp. 11–197. ISBN 9780841298941. [Google Scholar]
- Yadav, A.; Bagotia, N.; Sharma, A.K.; Kumar, S. Advances in decontamination of wastewater using biomass-basedcomposites: A critical review. Sci. Total Environ. 2021, 784, 147108. [Google Scholar] [CrossRef]
- Wen, Z.; Lu, J.; Zhang, Y.; Cheng, G.; Huang, S.; Chen, J.; Xu, R.; Ming, Y.-A.; Wang, Y.; Chen, R. Facile inverse micelle fabrication of magnetic ordered mesoporous iron cerium bimetal oxides with excellent performance for arsenic removal from water. J. Hazard. Mater. 2020, 383, 121172. [Google Scholar] [CrossRef] [PubMed]
- Srivastav, A.L.; Pham, T.D.; Izah, S.C.; Singh, N.; Singh, P.K. Biochar Adsorbents for Arsenic Removal from Water Environment: A Review. Bull. Environ. Contam. Toxicol. 2021, 1–13. [Google Scholar] [CrossRef]
- Mudzielwana, R.; Gitari, M.W.; Ndungu, P. Enhanced As(III) and As(V) Adsorption From Aqueous Solution by a Clay Based Hybrid Sorbent. Front. Chem. 2020, 7, 913. [Google Scholar] [CrossRef] [Green Version]
- Siddiq, O.M.; Tawabini, B.S.; Soupios, P.; Ntarlagiannis, D. Removal of arsenic from contaminated groundwater using biochar: A technical review. Int. J. Environ. Sci. Technol. 2022, 19, 651–664. [Google Scholar] [CrossRef]
- Manyà, J.J. Pyrolysis for Biochar Purposes: A Review to Establish Current Knowledge Gaps and Research Needs. Environ. Sci. Technol. 2012, 46, 7939–7954. [Google Scholar] [CrossRef]
- Bachmann, H.J.; Bucheli, T.D.; Dieguez-Alonso, A.; Fabbri, D.; Knicker, H.; Schmidt, H.-P.; Ulbricht, A.; Becker, R.; Buscaroli, A.; Buerge, D.; et al. Toward the Standardization of Biochar Analysis: The COST Action TD1107 Interlaboratory Comparison. J. Agric. Food Chem. 2016, 64, 513–527. [Google Scholar] [CrossRef]
- Guo, M.; Song, W.; Tian, J. Biochar-Facilitated Soil Remediation: Mechanisms and Efficacy Variations. Front. Environ. Sci. 2020, 8, 183. [Google Scholar] [CrossRef]
- Xiang, W.; Zhang, X.; Chen, J.; Zou, W.; He, F.; Hu, X.; Tsang, D.C.W.; Ok, Y.S.; Gao, B. Biochar technology in wastewater treatment: A critical review. Chemosphere 2020, 252, 126539. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Reddy, K.R.; Wang, C.; Yargicoglu, E.; Spokas, K. Characteristics and Applications of Biochar for Environmental Remediation: A Review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 939–969. [Google Scholar] [CrossRef]
- Chen, C.-K.; Chen, J.-J.; Nguyen, N.-T.; Le, T.-T.; Nguyen, N.-C.; Chang, C.-T. Specifically designed magnetic biochar from waste wood for arsenic removal. Sustain. Environ. Res. 2021, 31, 29. [Google Scholar] [CrossRef]
- Navarathna, C.M.; Karunanayake, A.G.; Gunatilake, S.R.; Pittman, C.U.; Perez, F.; Mohan, D.; Mlsna, T. Removal of Arsenic(III) from water using magnetite precipitated onto Douglas fir biochar. J. Environ. Manag. 2019, 250, 109429. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Lee, J.-H.; Lee, S.-L.; Hwang, S.-W.; Seo, D.-C. Adsorption behavior of arsenic onto lignin-based biochar decorated with zinc. Colloids Surfaces A Physicochem. Eng. Asp. 2021, 626, 127095. [Google Scholar] [CrossRef]
- Wang, X.; Guo, Z.; Hu, Z.; Zhang, J. Recent advances in biochar application for water and wastewater treatment: A review. PeerJ 2020, 8, e9164. [Google Scholar] [CrossRef]
- Faheem; Du, J.; Kim, S.H.; Hassan, M.A.; Irshad, S.; Bao, J. Application of biochar in advanced oxidation processes: Supportive, adsorptive, and catalytic role. Environ. Sci. Pollut. Res. 2020, 27, 37286–37312. [Google Scholar] [CrossRef]
- Pan, X.; Gu, Z.; Chen, W.; Li, Q. Preparation of biochar and biochar composites and their application in a Fenton-like process for wastewater decontamination: A review. Sci. Total Environ. 2021, 754, 142104. [Google Scholar] [CrossRef]
- Zubair, M.; Ihsanullah, I.; Aziz, H.A.; Ahmad, M.A.; Al-Harthi, M.A. Sustainable wastewater treatment by biochar/layered double hydroxide composites: Progress, challenges, and outlook. Bioresour. Technol. 2021, 319, 124128. [Google Scholar] [CrossRef]
- Duwiejuah, A.B.; Abubakari, A.H.; Quainoo, A.K.; Amadu, Y. Review of Biochar Properties and Remediation of Metal Pollution of Water and Soil. J. Health Pollut. 2020, 10, 200902. [Google Scholar] [CrossRef] [PubMed]
- Pourret, O.; Houben, D. Characterization of metal binding sites onto biochar using rare earth elements as a fingerprint. Heliyon 2018, 4, e00543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arevalo-Gallegos, A.; Ahmad, Z.; Asgher, M.; Parra-Saldivar, R.; Iqbal, H.M.N. Lignocellulose: A sustainable material to produce value-added products with a zero waste approach—A review. Int. J. Biol. Macromol. 2017, 99, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Rajapaksha, A.U.; Chen, S.S.; Tsang, D.C.W.; Zhang, M.; Vithanage, M.; Mandal, S.; Gao, B.; Bolan, N.S.; Ok, Y.S. Engineered/designer biochar for contaminant removal/immobilization from soil and water: Potential and implication of biochar modification. Chemosphere 2016, 148, 276–291. [Google Scholar] [CrossRef]
- Anae, J.; Ahmad, N.; Kumar, V.; Thakur, V.K.; Gutierrez, T.; Yang, X.J.; Cai, C.; Yang, Z.; Coulon, F. Recent advances in biochar engineering for soil contaminated with complex chemical mixtures: Remediation strategies and future perspectives. Sci. Total Environ. 2021, 767, 144351. [Google Scholar] [CrossRef]
- Wang, H.; Tian, Z.; Jiang, L.; Luo, W.; Wei, Z.; Li, S.; Cui, J.; Wei, W. Highly efficient adsorption of Cr(VI) from aqueous solution by Fe3+ impregnated biochar. J. Dispers. Sci. Technol. 2017, 38, 815–825. [Google Scholar] [CrossRef]
- Kumar, R.; Laskar, M.A.; Hewaidy, I.F.; Barakat, M.A. Modified Adsorbents for Removal of Heavy Metals from Aqueous Environment: A Review. Earth Syst. Environ. 2019, 3, 83–93. [Google Scholar] [CrossRef]
- Wu, C.; Cui, M.; Xue, S.; Li, W.; Huang, L.; Jiang, X.; Qian, Z. Remediation of arsenic-contaminated paddy soil by iron-modified biochar. Environ. Sci. Pollut. Res. 2018, 25, 20792–20801. [Google Scholar] [CrossRef]
- Godwin, P.M.; Pan, Y.; Xiao, H.; Afzal, M.T. Progress in Preparation and Application of Modified Biochar for Improving Heavy Metal Ion Removal From Wastewater. J. Bioresour. Bioprod. 2019, 4, 31–42. [Google Scholar] [CrossRef]
- Agrafioti, E.; Kalderis, D.; Diamadopoulos, E. Ca and Fe modified biochars as adsorbents of arsenic and chromium in aqueous solutions. J. Environ. Manag. 2014, 146, 444–450. [Google Scholar] [CrossRef]
- Georgaki, I.; Soupios, P.; Sakkas, N.; Ververidis, F.; Trantas, E.; Vallianatos, F.; Manios, T. Evaluating the use of electrical resistivity imaging technique for improving CH4 and CO2 emission rate estimations in landfills. Sci. Total Environ. 2008, 389, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Simyrdanis, K.; Papadopoulos, N.; Soupios, P.; Kirkou, S.; Tsourlos, P. Characterization and monitoring of subsurface contamination from Olive Oil Mills’ waste waters using Electrical Resistivity Tomography. Sci. Total Environ. 2018, 637–638, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Sparrenbom, C.J.; Åkesson, S.; Johansson, S.; Hagerberg, D.; Dahlin, T. Investigation of chlorinated solvent pollution with resistivity and induced polarization. Sci. Total Environ. 2017, 575, 767–778. [Google Scholar] [CrossRef] [PubMed]
- Kessouri, P.; Furman, A.; Huisman, J.A.; Martin, T.; Mellage, A.; Ntarlagiannis, D.; Bücker, M.; Ehosioke, S.; Fernandez, P.; Flores-Orozco, A.; et al. Induced polarization applied to biogeophysics: Recent advances and future prospects. Near Surf. Geophys. 2019, 17, 595–621. [Google Scholar] [CrossRef]
- Kimak, C.; Ntarlagiannis, D.; Slater, L.D.; Atekwana, E.A.; Beaver, C.L.; Rossbach, S.; Porter, A.; Ustra, A. Geophysical Monitoring of Hydrocarbon Biodegradation in Highly Conductive Environments. J. Geophys. Res. Biogeosci. 2019, 124, 353–366. [Google Scholar] [CrossRef]
- Ntarlagiannis, D.; Doherty, R.; Williams, K.H. Spectral induced polarization signatures of abiotic FeS precipitation. Geophysics 2010, 75, F127–F133. [Google Scholar] [CrossRef] [Green Version]
- Ntarlagiannis, D.; Kirmizakis, P.; Kalderis, D.; Soupios, P. Using the spectral induced polarization method to assess biochar performance as a remediation agent. In Proceedings of the AGU Fall Meeting, San Francisco, CA, USA, 12–16 December 2016. [Google Scholar]
- Kemna, A.; Binley, A.; Cassiani, G.; Niederleithinger, E.; Revil, A.; Slater, L.; Williams, K.H.; Orozco, A.F.; Haegel, F.-H.; Hördt, A.; et al. An overview of the spectral induced polarization method for near-surface applications. Near Surf. Geophys. 2012, 10, 453–468. [Google Scholar] [CrossRef] [Green Version]
- Kirmizakis, P.; Kalderis, D.; Ntarlagiannis, D.; Soupios, P. Preliminary assessment on the application of biochar and spectral-induced polarization for wastewater treatment. Near Surf. Geophys. 2020, 18, 109–122. [Google Scholar] [CrossRef]
- Ben Salem, I.; El Gamal, M.; Sharma, M.; Hameedi, S.; Howari, F.M. Utilization of the UAE date palm leaf biochar in carbon dioxide capture and sequestration processes. J. Environ. Manag. 2021, 299, 113644. [Google Scholar] [CrossRef]
- Usman, A.R.A.; Abduljabbar, A.; Vithanage, M.; Ok, Y.S.; Ahmad, M.; Ahmad, M.; Elfaki, J.; Abdulazeem, S.S.; Al-Wabel, M.I. Biochar production from date palm waste: Charring temperature induced changes in composition and surface chemistry. J. Anal. Appl. Pyrolysis 2015, 115, 392–400. [Google Scholar] [CrossRef]
- Din, M.I.; Raza, M.; Hussain, Z.; Mehmood, H.A. Fabrication of magnetite nanoparticles (Fe3O4-NPs) for catalytic pyrolysis of nutshells biomass. Soft Mater. 2019, 17, 24–31. [Google Scholar] [CrossRef]
- Slater, L.D.; Lesmes, D. IP interpretation in environmental investigations. Geophysics 2002, 67, 77–88. [Google Scholar] [CrossRef]
- Binley, A.; Kemna, A. DC Resistivity and Induced Polarization Methods. Hydrogeophysics 2005, 50, 129–156. [Google Scholar] [CrossRef]
- Xu, Y.; Xie, X.; Feng, Y.; Ashraf, M.A.; Liu, Y.; Su, C.; Qian, K.; Liu, P. As(III) and As(V) removal mechanisms by Fe-modified biochar characterized using synchrotron-based X-ray absorption spectroscopy and confocal micro-X-ray fluorescence imaging. Bioresour. Technol. 2020, 304, 122978. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Cao, X.; Mašek, O.; Zimmerman, A. Heterogeneity of biochar properties as a function of feedstock sources and production temperatures. J. Hazard. Mater. 2013, 256–257, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Usman, A.; Sallam, A.; Zhang, M.; Vithanage, M.; Ahmad, M.; Al-Farraj, A.; Ok, Y.S.; Abduljabbar, A.; Al-Wabel, M. Sorption Process of Date Palm Biochar for Aqueous Cd (II) Removal: Efficiency and Mechanisms. Water Air Soil Pollut. 2016, 227, 449. [Google Scholar] [CrossRef]
- Zhao, Z.; Wu, Q.; Nie, T.; Zhou, W. Quantitative evaluation of relationships between adsorption and partition of atrazine in biochar-amended soils with biochar characteristics. RSC Adv. 2019, 9, 4162–4171. [Google Scholar] [CrossRef] [Green Version]
- Sackey, E.A.; Song, Y.; Yu, Y.; Zhuang, H. Biochars derived from bamboo and rice straw for sorption of basic red dyes. PLoS ONE 2021, 16, e0254637. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic Molecular Structure of Plant Biomass-Derived Black Carbon (Biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Liu, H.; Sun, Z.; Huang, J.; Liao, Y. Supported Nanosized α-FeOOH Improves Efficiency of Photoelectro-Fenton Process with Reaction-Controlled pH Adjustment for Sustainable Water Treatment. Int. J. Photoenergy 2012, 2012, 689807. [Google Scholar] [CrossRef] [Green Version]
- Pongkua, W.; Dolphen, R.; Thiravetyan, P. Effect of functional groups of biochars and their ash content on gaseous methyl tert-butyl ether removal. Colloids Surf. A Physicochem. Eng. Asp. 2018, 558, 531–537. [Google Scholar] [CrossRef]
- Racek, J.; Sevcik, J.; Chorazy, T.; Kucerik, J.; Hlavinek, P. Biochar—Recovery Material from Pyrolysis of Sewage Sludge: A Review. Waste Biomass Valorization 2020, 11, 3677–3709. [Google Scholar] [CrossRef]
- Sizmur, T.; Fresno, T.; Akgül, G.; Frost, H.; Moreno-Jiménez, E. Biochar modification to enhance sorption of inorganics from water. Bioresour. Technol. 2017, 246, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Benis, K.Z.; Damuchali, A.M.; Soltan, J.; McPhedran, K.N. Treatment of aqueous arsenic—A review of biochar modification methods. Sci. Total Environ. 2020, 739, 139750. [Google Scholar] [CrossRef]
- Kumar, R.; Kang, C.-U.; Mohan, D.; Khan, M.A.; Lee, J.-H.; Lee, S.S.; Jeon, B.-H. Waste sludge derived adsorbents for arsenate removal from water. Chemosphere 2020, 239, 124832. [Google Scholar] [CrossRef]
- Niazi, N.K.; Bibi, I.; Shahid, M.; Ok, Y.S.; Burton, E.D.; Wang, H.; Shaheen, S.M.; Rinklebe, J.; Lüttge, A. Arsenic removal by perilla leaf biochar in aqueous solutions and groundwater: An integrated spectroscopic and microscopic examination. Environ. Pollut. 2018, 232, 31–41. [Google Scholar] [CrossRef]
- Luengo, C.; Puccia, V.; Avena, M. Arsenate adsorption and desorption kinetics on a Fe(III)-modified montmorillonite. J. Hazard. Mater. 2011, 186, 1713–1719. [Google Scholar] [CrossRef]
- Godlewska, P.; Bogusz, A.; Dobrzyńska, J.; Dobrowolski, R.; Oleszczuk, P. Engineered biochar modified with iron as a new adsorbent for treatment of water contaminated by selenium. J. Saudi Chem. Soc. 2020, 24, 824–834. [Google Scholar] [CrossRef]
- Dzade, N.Y.; Roldan, A.; De Leeuw, N.H. Structures and Properties of As(OH)3 Adsorption Complexes on Hydrated Mackinawite (FeS) Surfaces: A DFT-D2 Study. Environ. Sci. Technol. 2017, 51, 3461–3470. [Google Scholar] [CrossRef] [Green Version]
- Coates, J. Interpretation of Infrared Spectra, a Practical Approach. In Encyclopedia of Analytical Chemistry; John Wiley & Sons: Chichester, UK, 2006; Volume 112, pp. 22–31. [Google Scholar]
- Sattar, M.S.; Shakoor, M.B.; Ali, S.; Rizwan, M.; Niazi, N.K.; Jilani, A. Comparative efficiency of peanut shell and peanut shell biochar for removal of arsenic from water. Environ. Sci. Pollut. Res. 2019, 26, 18624–18635. [Google Scholar] [CrossRef]
- Çatlıoğlu, F.; Akay, S.; Turunç, E.; Gözmen, B.; Anastopoulos, I.; Kayan, B.; Kalderis, D. Preparation and application of Fe-modified banana peel in the adsorption of methylene blue: Process optimization using response surface methodology. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100517. [Google Scholar] [CrossRef]
- Alchouron, J.; Navarathna, C.; Chludil, H.D.; Dewage, N.B.; Perez, F.; Hassan, E.B.; Pittman, C.U., Jr.; Vega, A.S.; Mlsna, T.E. Assessing South American Guadua chacoensis bamboo biochar and Fe3O4 nanoparticle dispersed analogues for aqueous arsenic(V) remediation. Sci. Total Environ. 2020, 706, 135943. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Gao, B.; Zimmerman, A.R.; Li, Y.; Ma, L.; Harris, W.G.; Migliaccio, K.W. Removal of arsenic by magnetic biochar prepared from pinewood and natural hematite. Bioresour. Technol. 2015, 175, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Ding, Z.; Zimmerman, A.R.; Wang, S.; Gao, B. Batch and column sorption of arsenic onto iron-impregnated biochar synthesized through hydrolysis. Water Res. 2015, 68, 206–216. [Google Scholar] [CrossRef]
- Bakshi, S.; Banik, C.; Rathke, S.J.; Laird, D.A. Arsenic sorption on zero-valent iron-biochar complexes. Water Res. 2018, 137, 153–163. [Google Scholar] [CrossRef]
- Wen, T.; Wang, J.; Yu, S.; Chen, Z.; Hayat, T.; Wang, X. Magnetic Porous Carbonaceous Material Produced from Tea Waste for Efficient Removal of As(V), Cr(VI), Humic Acid, and Dyes. ACS Sustain. Chem. Eng. 2017, 5, 4371–4380. [Google Scholar] [CrossRef]
- Fan, J.; Xu, X.; Ni, Q.; Lin, Q.; Fang, J.; Chen, Q.; Shen, X.; Lou, L. Enhanced As (V) Removal from Aqueous Solution by Biochar Prepared from Iron-Impregnated Corn Straw. J. Chem. 2018, 2018, 5137694. [Google Scholar] [CrossRef] [Green Version]
- Fan, Q.; Sun, J.; Chu, L.; Cui, L.; Quan, G.; Yan, J.; Hussain, Q.; Iqbal, M. Effects of chemical oxidation on surface oxygen-containing functional groups and adsorption behavior of biochar. Chemosphere 2018, 207, 33–40. [Google Scholar] [CrossRef]
- Merriam, J.B. Induced polarization and surface electrochemistry. Geophysics 2007, 72, F157–F166. [Google Scholar] [CrossRef]
- Titov, K.; Komarov, V.; Tarasov, V.; Levitski, A. Theoretical and experimental study of time domain-induced polarization in water-saturated sands. J. Appl. Geophys. 2002, 50, 417–433. [Google Scholar] [CrossRef]
- Vinegar, H.J.; Waxman, M.H. Induced polarization of shaly sands. Geophysics 1984, 49, 1267–1287. [Google Scholar] [CrossRef]







| Sample | Yield (%) | Ash (%) | C (wt. %) | H (wt. %) | N (wt. %) | S (wt. %) | O 1 (wt. %) | Atomic H/C | Atomic O/C | Atomic (N+O)/C |
|---|---|---|---|---|---|---|---|---|---|---|
| Date palm leaves | - | 8 | 48.97 | 5.28 | 2.89 | 0.56 | 31.16 | 1.29 | 0.47 | 0.52 |
| BC-500 | 79 | 11.7 | 50.19 | 2.80 | 1.80 | 0.26 | 33.51 | 0.66 | 0.49 | 0.53 |
| BC-800 | 48 | 26 | 55.40 | 1.12 | 1.12 | 0.0 | 30.95 | 0.24 | 0.41 | 0.43 |
| Adsorbent | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| Qm (mg/g) | KL (Lg−1) | R2 | n | KF (mg1-ng−1Ln) | R2 | |
| Fe-BC-500 | 0.962 | 3.42 | 0.85 | 54.3 | 6.91 | 0.89 |
| Qm (mg/g) values of other adsorbents | ||||||
| Fe-bamboo biochar, pH 7, 25 °C [76] | 49 | Magnetic pinewood biochar, pH 7 [77] | 0.3 | |||
| Fe-hickory biochar, pH 5.8 [78] | 22 | Zero valent iron switchgrass biochar, pH 7–7.5 [79] | 7.9 | |||
| Tea waste magnetic porous carbonaceous, pH 5 [80] | 38 | Iron impregnated biochar, pH 3 [81] | 14.7 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirmizakis, P.; Tawabini, B.; Siddiq, O.M.; Kalderis, D.; Ntarlagiannis, D.; Soupios, P. Adsorption of Arsenic on Fe-Modified Biochar and Monitoring Using Spectral Induced Polarization. Water 2022, 14, 563. https://doi.org/10.3390/w14040563
Kirmizakis P, Tawabini B, Siddiq OM, Kalderis D, Ntarlagiannis D, Soupios P. Adsorption of Arsenic on Fe-Modified Biochar and Monitoring Using Spectral Induced Polarization. Water. 2022; 14(4):563. https://doi.org/10.3390/w14040563
Chicago/Turabian StyleKirmizakis, Panagiotis, Bassam Tawabini, Omer Muhammad Siddiq, Dimitrios Kalderis, Dimitrios Ntarlagiannis, and Pantelis Soupios. 2022. "Adsorption of Arsenic on Fe-Modified Biochar and Monitoring Using Spectral Induced Polarization" Water 14, no. 4: 563. https://doi.org/10.3390/w14040563