Zerovalent Iron Nanoparticles-Alginate Nanocomposites for Cr(VI) Removal in Water—Influence of Temperature, pH, Dissolved Oxygen, Matrix, and nZVI Surface Composition
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
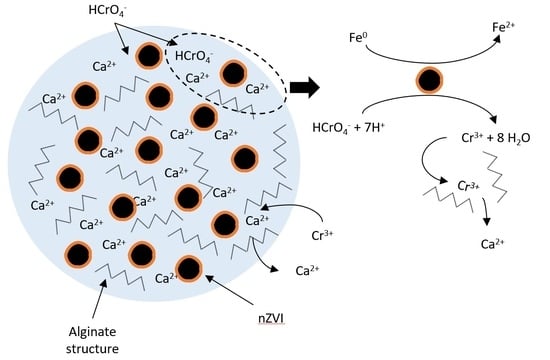
2.2. Synthesis and Characterization of nZVI@AL Beads
2.3. Chromium Removal
2.4. Analytical Methods
3. Results
3.1. SEM-EDS, XRD, Raman Analysis
3.2. Cr(VI) Removal
3.2.1. Effect of pH, Dissolved Oxygen, Temperature, and Alginate Viscosity
3.2.2. N25 vs. NSTAR
3.2.3. Chromium Removal and Speciation Using NSTAR@AL
4. Discussion
4.1. Impact of Immobilization on nZVI Physicochemical Identity
4.2. Role of the Polymer Matrix in Cr(VI) Removal
4.3. nZVI Physicochemical Identity and Cr(VI) Removal
4.4. Retention of Cr(III)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Alazaiza, M.Y.D.; Albahnasawi, A.; Ali, G.A.M.; Bashir, M.J.K.; Copty, N.K.; Amr, S.S.A.; Abushammala, M.F.M.; Al Maskari, T. Recent Advances of Nanoremediation Technologies for Soil and Groundwater Remediation: A Review. Water 2021, 13, 2186. [Google Scholar] [CrossRef]
- Crane, R.A.; Scott, T.B. Nanoscale zero-valent iron: Future prospects for an emerging water treatment technology. J. Hazard. Mater. 2012, 211–212, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Scott, T.B.; Popescu, I.C.; Crane, R.A.; Noubactep, C. Nano-scale metallic iron for the treatment of solutions containing multiple inorganic contaminants. J. Hazard. Mater. 2011, 186, 280–287. [Google Scholar] [CrossRef]
- Yan, W.; Herzing, A.A.; Kiely, C.J.; Zhang, W. Nanoscale zero-valent iron (nZVI): Aspects of the core-shell structure and reactions with inorganic species in water. J. Contam. Hydrol. 2010, 118, 96–104. [Google Scholar] [CrossRef]
- Wang, P.; Fu, F.; Liu, T. A review of the new multifunctional nano zero-valent iron composites for wastewater treatment: Emergence, preparation, optimization and mechanism. Chemosphere 2021, 285, 131435. [Google Scholar] [CrossRef]
- Xie, X.; Wang, Y.; Pi, K.; Liu, C.; Li, J.; Liu, Y.; Wang, Z.; Duan, M. In situ treatment of arsenic contaminated groundwater by aquifer iron coating: Experimental study. Sci. Total Environ. 2015, 527–528, 38–46. [Google Scholar] [CrossRef] [Green Version]
- Adeleye, A.S.; Conway, J.R.; Garner, K.; Huang, Y.; Su, Y.; Keller, A.A. Engineered nanomaterials for water treatment and remediation: Costs, benefits, and applicability. Chem. Eng. J. 2016, 286, 640–662. [Google Scholar] [CrossRef] [Green Version]
- Yan, W.; Lien, H.-L.; Koel, B.E.; Zhang, W. Iron nanoparticles for environmental clean-up: Recent developments and future outlook. Environ. Sci. Process. Impacts 2013, 15, 63. [Google Scholar] [CrossRef] [PubMed]
- Ribas, D.; Černík, M.; Benito, J.A.A.; Filip, J.; Marti, V. Activation process of air stable nanoscale zero-valent iron particles. Chem. Eng. J. 2017, 320, 290–299. [Google Scholar] [CrossRef] [Green Version]
- Nahuel Montesinos, V.; Quici, N.; Beatriz Halac, E.; Leyva, A.G.; Custo, G.; Bengio, S.; Zampieri, G.; Litter, M.I. Highly efficient removal of Cr(VI) from water with nanoparticulated zerovalent iron: Understanding the Fe(III)-Cr(III) passive outer layer structure. Chem. Eng. J. 2014, 244, 569–575. [Google Scholar] [CrossRef]
- Sharma, V.K.; Filip, J.; Zboril, R.; Varma, R.S. Natural inorganic nanoparticles-formation, fate, and toxicity in the environment. Chem. Soc. Rev. 2015, 44, 8410–8423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasarevičius, S.; Danila, V.; Januševičius, T. Immobilisation of Cadmium, Copper, Lead, and Nickel in Soil Using Nano Zerovalent Iron Particles: Ageing Effect on Heavy Metal Retention. Water Air Soil Pollut. 2020, 231, 496. [Google Scholar] [CrossRef]
- Mortazavian, S.; An, H.; Chun, D.; Moon, J. Activated carbon impregnated by zero-valent iron nanoparticles (AC/nZVI) optimized for simultaneous adsorption and reduction of aqueous hexavalent chromium: Material characterizations and kinetic studies. Chem. Eng. J. 2018, 353, 781–795. [Google Scholar] [CrossRef]
- Liu, X.; Lai, D.; Wang, Y. Performance of Pb(II) removal by an activated carbon supported nanoscale zero-valent iron composite at ultralow iron content. J. Hazard. Mater. 2019, 361, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W.; Johir, M.A.H.; Sornalingam, K.; Belhaj, D.; Kallel, M. Nano-Fe0 immobilized onto functionalized biochar gaining excellent stability during sorption and reduction of chloramphenicol via transforming to reusable magnetic composite. Chem. Eng. J. 2017, 322, 571–581. [Google Scholar] [CrossRef]
- Su, H.; Fang, Z.; Tsang, P.E.; Zheng, L.; Cheng, W.; Fang, J.; Zhao, D. Remediation of hexavalent chromium contaminated soil by biochar-supported zero-valent iron nanoparticles. J. Hazard. Mater. 2016, 318, 533–540. [Google Scholar] [CrossRef]
- Li, Z.; Sun, Y.; Yang, Y.; Han, Y.; Wang, T.; Chen, J.; Tsang, D.C.W. Biochar-supported nanoscale zero-valent iron as an efficient catalyst for organic degradation in groundwater. J. Hazard. Mater. 2020, 383, 121240. [Google Scholar] [CrossRef]
- Vázquez-Guerrero, A.; Cortés-Martínez, R.; Alfaro-Cuevas-Villanueva, R.; Rivera-Muñoz, E.; Huirache-Acuña, R. Cd(II) and Pb(II) Adsorption Using a Composite Obtained from Moringa oleifera Lam. Cellulose Nanofibrils Impregnated with Iron Nanoparticles. Water 2021, 13, 89. [Google Scholar] [CrossRef]
- Ahmed, M.F.; Abbas, M.A.; Mahmood, A.; Ahmad, N.M.; Rasheed, H.; Qadir, M.A.; Khan, A.U.; Qiblawey, H.; Zhu, S.; Sadiq, R.; et al. Hybrid Beads of Zero Valent Iron Oxide Nanoparticles and Chitosan for Removal of Arsenic in Contaminated Water. Water 2021, 13, 2876. [Google Scholar] [CrossRef]
- Sciscenko, I.; Luca, V.; Ramos, C.P.; Scott, T.B.; Montesinos, V.N.; Quici, N. Immobilization of nanoscale zerovalent iron in hierarchically channelled polyacrylonitrile for Cr(VI) remediation in wastewater. J. Water Process Eng. 2021, 39, 101704. [Google Scholar] [CrossRef]
- Bezbaruah, A.N.; Shanbhogue, S.S.; Simsek, S.; Khan, E. Encapsulation of iron nanoparticles in alginate biopolymer for trichloroethylene remediation. J. Nanoparticle Res. 2011, 13, 6673–6681. [Google Scholar] [CrossRef]
- Ravikumar, K.V.G.; Kumar, D.; Kumar, G.; Mrudula, P.; Natarajan, C.; Mukherjee, A. Enhanced Cr(VI) Removal by Nanozerovalent Iron-Immobilized Alginate Beads in the Presence of a Biofilm in a Continuous-Flow Reactor. Ind. Eng. Chem. Res. 2016, 55, 5973–5982. [Google Scholar] [CrossRef]
- Andreia, C.; Pereira, A.; Nava, M.R.; Walter, J.B.; Scherer, C.E.; Dominique, A.; Dalfovo, K.; Barreto-rodrigues, M. Application of zero valent iron ( ZVI ) immobilized in Ca-Alginate beads for CI Reactive Red 195 catalytic degradation in an air lift reactor operated with ozone. J. Hazard. Mater. 2021, 401, 123275. [Google Scholar] [CrossRef]
- Tesh, S.J.; Scott, T.B. Nano-Composites for Water Remediation: A Review. Adv. Mater. 2014, 26, 6056–6068. [Google Scholar] [CrossRef] [PubMed]
- Bezbaruah, A.N.; Kalita, H.; Almeelbi, T.; Capecchi, C.L.; Jacob, D.L.; Ugrinov, A.G.; Payne, S.A. Ca–alginate-entrapped nanoscale iron: Arsenic treatability and mechanism studies. J. Nanoparticle Res. 2014, 16, 2175. [Google Scholar] [CrossRef]
- Bezbaruah, A.N.; Krajangpan, S.; Chisholm, B.J.; Khan, E.; Elorza Bermudez, J.J. Entrapment of iron nanoparticles in calcium alginate beads for groundwater remediation applications. J. Hazard. Mater. 2009, 166, 1339–1343. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Wan, Y.; Zheng, Y.; Lee, X.; Liu, T.; Yu, Z.; Huang, J.; Ok, Y.S.; Chen, J.; Gao, B. Alginate-based composites for environmental applications: A critical review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 318–356. [Google Scholar] [CrossRef] [PubMed]
- Owlad, M.; Aroua, M.K.; Daud, W.A.W.; Baroutian, S. Removal of Hexavalent Chromium-Contaminated Water and Wastewater: A Review. Water. Air. Soil Pollut. 2009, 200, 59–77. [Google Scholar] [CrossRef]
- Coetzee, J.J.; Bansal, N.; Chirwa, E.M.N. Chromium in Environment, Its Toxic Effect from Chromite-Mining and Ferrochrome Industries, and Its Possible Bioremediation. Expo. Heal. 2020, 12, 51–62. [Google Scholar] [CrossRef] [Green Version]
- GracePavithra, K.; Jaikumar, V.; Kumar, P.S.; SundarRajan, P. A review on cleaner strategies for chromium industrial wastewater: Present research and future perspective. J. Clean. Prod. 2019, 228, 580–593. [Google Scholar] [CrossRef]
- Hausladen, D.M.; Alexander-Ozinskas, A.; McClain, C.; Fendorf, S. Hexavalent Chromium Sources and Distribution in California Groundwater. Environ. Sci. Technol. 2018, 52, 8242–8251. [Google Scholar] [CrossRef] [PubMed]
- Zhitkovich, A. Chromium in drinking water: Sources, metabolism, and cancer risks. Chem. Res. Toxicol. 2011, 24, 1617–1629. [Google Scholar] [CrossRef] [PubMed]
- Word Health Organization Chromium in Drinking-Water Background Document for Development of WHO Guidelines for Drinking-Water Quality. Available online: http://linkinghub.elsevier.com/retrieve/pii/S1607551X11001112 (accessed on 17 October 2021).
- Brown, E.G.; Alexeeff, G.V. Public Health Goals for Chemicals in Drinking Water. Available online: https://oehha.ca.gov/media/downloads/water/chemicals/phg/cr6phg072911.pdf (accessed on 15 October 2021).
- Montesinos, V.N.; Quici, N.; Litter, M.I. Visible light enhanced Cr(VI) removal from aqueous solution by nanoparticulated zerovalent iron. Catal. Commun. 2014, 46, 57–60. [Google Scholar] [CrossRef]
- Quici, N.; Meichtry, M.; Montesinos, V.N. Use of Nanoparticulated Iron Materials for Chromium, Arsenic, and Uranium Removal from Water. In Iron Nanomaterials for Water and Soil Treatment; Marta I, L., Quici, N., Meichtry, J.M., Eds.; Pan Stanford Publishing: Singapore, 2018; pp. 177–188. ISBN 9789814774673. [Google Scholar]
- Liu, T.; Zhao, L.; Sun, D.; Tan, X. Entrapment of nanoscale zero-valent iron in chitosan beads for hexavalent chromium removal from wastewater. J. Hazard. Mater. 2010, 184, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Cao, J.; Zhang, W. Stoichiometry of Cr(VI) immobilization using nanoscale zerovalent iron (nZVI): A study with high-resolution X-ray photoelectron spectroscopy (HR-XPS). Ind. Eng. Chem. Res. 2008, 47, 2131–2139. [Google Scholar] [CrossRef]
- Vilardi, G.; Mpouras, T.; Dermatas, D.; Verdone, N.; Polydera, A.; Di Palma, L. Nanomaterials application for heavy metals recovery from polluted water: The combination of nano zero-valent iron and carbon nanotubes. Competitive adsorption non-linear modeling. Chemosphere 2018, 201, 716–729. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Ma, J.; Xie, L.; Tang, B.; Han, W.; Lin, S. Chromium removal using resin supported nanoscale zero-valent iron. J. Environ. Manage. 2013, 128, 822–827. [Google Scholar] [CrossRef]
- Li, X.; Ai, L.; Jiang, J. Nanoscale zerovalent iron decorated on graphene nanosheets for Cr(VI) removal from aqueous solution: Surface corrosion retard induced the enhanced performance. Chem. Eng. J. 2016, 288, 789–797. [Google Scholar] [CrossRef]
- Liu, P.; Wang, X.; Ma, J.; Liu, H.; Ning, P. Highly efficient immobilization of NZVI onto bio-inspired reagents functionalized polyacrylonitrile membrane for Cr(VI) reduction. Chemosphere 2019, 220, 1003–1013. [Google Scholar] [CrossRef]
- ASTM Standard Test Methods for Chromium in Water (D 1687-92). In Annual Book of ASTM Standards; ASTM International: Filadelfia, PA, USA, 1999.
- Filip, J.; Karlický, F.; Marušák, Z.; Lazar, P.; Černík, M.; Otyepka, M.; Zbořil, R. Anaerobic Reaction of Nanoscale Zerovalent Iron with Water: Mechanism and Kinetics. J. Phys. Chem. C 2014, 118, 13817–13825. [Google Scholar] [CrossRef]
- Liu, A.; Liu, J.; Pan, B.; Zhang, W.X. Formation of lepidocrocite (γ-FeOOH) from oxidation of nanoscale zero-valent iron (nZVI) in oxygenated water. RSC Adv. 2014, 4, 57377–57382. [Google Scholar] [CrossRef]
- Schwertmann, U.; Fechter, H. The Formation of Green Rust and Its Transformation to Lepidocrocite. Clay Miner. 1994, 29, 87–92. [Google Scholar] [CrossRef] [Green Version]
- Liu, A.; Liu, J.; Han, J.; Zhang, W.X. Evolution of nanoscale zero-valent iron (nZVI) in water: Microscopic and spectroscopic evidence on the formation of nano- and micro-structured iron oxides. J. Hazard. Mater. 2017, 322, 129–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kašlík, J.; Kolařík, J.; Filip, J.; Medřík, I.; Tomanec, O.; Petr, M.; Malina, O.; Zbořil, R.; Tratnyek, P.G. Nanoarchitecture of advanced core-shell zero-valent iron particles with controlled reactivity for contaminant removal. Chem. Eng. J. 2018, 354, 335–345. [Google Scholar] [CrossRef]
- Huang, J.-F.; Li, Y.-T.; Wu, J.-H.; Cao, P.-Y.; Liu, Y.-L.; Jiang, G.-B. Floatable, macroporous structured alginate sphere supporting iron nanoparticles used for emergent Cr(VI) spill treatment. Carbohydr. Polym. 2016, 146, 115–122. [Google Scholar] [CrossRef]
- Lei, Z.; Zhai, S.; Lv, J.; Fan, Y.; An, Q.; Xiao, Z. Sodium alginate-based magnetic carbonaceous biosorbents for highly efficient Cr(VI) removal from water. RSC Adv. 2015, 5, 77932–77941. [Google Scholar] [CrossRef]
- Narayanan, R.P.; Melman, G.; Letourneau, N.J.; Mendelson, N.L.; Melman, A. Photodegradable Iron(III) Cross-Linked Alginate Gels. Biomacromolecules 2012, 13, 2465–2471. [Google Scholar] [CrossRef]
- Paul, U.C.; Manian, A.P.; Široká, B.; Duelli, H.; Bechtold, T. Sorption of iron(III)-alginate complexes on cellulose fibres. Cellulose 2013, 20, 2481–2490. [Google Scholar] [CrossRef]
- Xie, Y.; Cwiertny, D.M. Influence of anionic cosolutes and ph on nanoscale zerovalent iron longevity: Time scales and mechanisms of reactivity loss toward 1,1,1,2-tetrachloroethane and Cr(VI). Environ. Sci. Technol. 2012, 46, 8365–8373. [Google Scholar] [CrossRef]
- Sohn, K.; Kang, S.W.; Ahn, S.; Woo, M.; Yang, S.K. Fe(0) nanoparticles for nitrate reduction: Stability, reactivity, and transformation. Environ. Sci. Technol. 2006, 40, 5514–5519. [Google Scholar] [CrossRef]
- Schmid, D.; Micić, V.; Laumann, S.; Hofmann, T. Measuring the reactivity of commercially available zero-valent iron nanoparticles used for environmental remediation with iopromide. J. Contam. Hydrol. 2015, 181, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Jiang, G.; Xue, X.; Wu, D.; Sheng, T.; Sun, C.; Xu, X. Fe0-Fe3O4 nanocomposites embedded polyvinyl alcohol/sodium alginate beads for chromium (VI) removal. J. Hazard. Mater. 2013, 262, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Xu, J.; Jiang, G.; Tang, J.; Xu, X. Highly active nanoscale zero-valent iron (nZVI)–Fe3O4 nanocomposites for the removal of chromium(VI) from aqueous solutions. J. Colloid Interface Sci. 2012, 369, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Ravikumar, K.V.G.; Kumar, D.; Rajeshwari, A.; Madhu, G.M.; Mrudula, P.; Chandrasekaran, N.; Mukherjee, A. A comparative study with biologically and chemically synthesized nZVI: Applications in Cr (VI) removal and ecotoxicity assessment using indigenous microorganisms from chromium-contaminated site. Environ. Sci. Pollut. Res. 2016, 23, 2613–2627. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Kim, H. Preparation and application of sodium borohydride composites for portable hydrogen production. Energy 2010, 35, 960–963. [Google Scholar] [CrossRef]
- Simeonidis, K.; Tziomaki, M.; Angelakeris, M.; Martinez-Boubeta, C.; Balcells, L.; Monty, C.; Mitrakas, M.; Vourlias, G.; Andritsos, N. Development of iron-based nanoparticles for Cr(VI) removal from drinking water. EPJ Web Conf. 2013, 40, 08007. [Google Scholar] [CrossRef] [Green Version]
- Mu, Y.; Jia, F.; Ai, Z.; Zhang, L. Iron oxide shell mediated environmental remediation properties of nano zero-valent iron. Environ. Sci. Nano 2017, 4, 27–45. [Google Scholar] [CrossRef]
- Ravikumar, K.V.G.; Sudakaran, S.V.; Pulimi, M.; Natarajan, C.; Mukherjee, A. Removal of hexavalent chromium using nano zero valent iron and bacterial consortium immobilized alginate beads in a continuous flow reactor. Environ. Technol. Innov. 2018, 12, 104–114. [Google Scholar] [CrossRef]
- Yu, C.; Zhang, Y.; Fang, Y.; Tan, Y.; Dai, K.; Liu, S.; Huang, Q. Shewanella oneidensis MR-1 impregnated Ca-alginate capsule for efficient Cr(VI) reduction and Cr(III) adsorption. Environ. Sci. Pollut. Res. 2020, 27, 16745–16753. [Google Scholar] [CrossRef]







| Reference | Material | pH | Removal Capacity (mg Cr(VI) g nZVI−1) | Contact Time (h) | Immobilization Procedure |
|---|---|---|---|---|---|
| Huang et al. [49] | nFe@AL | 3 | 142.41 | 50 | Infiltration of Fe(III) ions in alginate beads, followed by reduction with . |
| Ravikumar et al. [58] | nFe@AL | 7 | 8.108 | 67.5 | Immobilization of nZVI previously synthesized by reduction of Fe(II) with . Filtrated and dryed at 100 °C. |
| Lv et al. [56] | nFe/F3O4@PVA/AL | 5 | 666* | 24 | Immobilization of synthesized nZVI by reduction with , combined with nanoparticles, followed by stabilization of 12 h in . Re-reduction with after immobilization. |
| This work | nFe@AL | 3 | 133 | 24 | Immobilization of commercial nZVI in AL. 4 h of hardening |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parnis, M.; García, F.E.; Toledo, M.V.; Montesinos, V.N.; Quici, N. Zerovalent Iron Nanoparticles-Alginate Nanocomposites for Cr(VI) Removal in Water—Influence of Temperature, pH, Dissolved Oxygen, Matrix, and nZVI Surface Composition. Water 2022, 14, 484. https://doi.org/10.3390/w14030484
Parnis M, García FE, Toledo MV, Montesinos VN, Quici N. Zerovalent Iron Nanoparticles-Alginate Nanocomposites for Cr(VI) Removal in Water—Influence of Temperature, pH, Dissolved Oxygen, Matrix, and nZVI Surface Composition. Water. 2022; 14(3):484. https://doi.org/10.3390/w14030484
Chicago/Turabian StyleParnis, Marguerite, Fabiana Elena García, Melanie Victoria Toledo, Víctor Nahuel Montesinos, and Natalia Quici. 2022. "Zerovalent Iron Nanoparticles-Alginate Nanocomposites for Cr(VI) Removal in Water—Influence of Temperature, pH, Dissolved Oxygen, Matrix, and nZVI Surface Composition" Water 14, no. 3: 484. https://doi.org/10.3390/w14030484