Degradation of Reactive Brilliant Red X-3B by Photo-Fenton-like Process: Effects of Water Chemistry Factors and Degradation Mechanism
Abstract
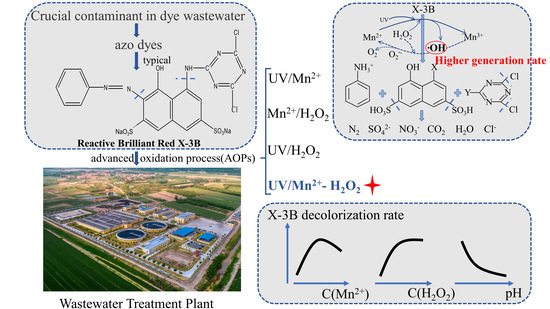
:1. Introduction
2. Materials and Methods
2.1. Preparation and Testing of Dye Wastewater
2.2. Experimental Apparatus and Procedures
2.3. Analytical Methods
3. Results and Discussion
3.1. Decolorization of X-3B by H2O2 with Different Catalysts
3.2. Degradation of X-3B in Different Systems
3.3. Factors Affecting the Degradation System of UV/Mn2+-H2O2
3.3.1. Effects of Mn2+ and H2O2 Concentrations
3.3.2. Effect of Initial Solution pH
3.3.3. Effect of Initial X-3B Concentration
3.4. Role of ∙OH in the Degradation System of UV/Mn2+-H2O2
3.5. Degradation Mechanism of UV/Mn2+-H2O2 System
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pan, F.; Yu, Y.; Xu, A.; Xia, D.; Sun, Y.; Cai, Z.; Liu, W.; Fu, J. Application of magnetic OMS-2 in sequencing batch reactor for treating dye wastewater as a modulator of microbial community. J. Hazard. Mater. 2017, 340, 36–46. [Google Scholar] [CrossRef]
- Samsami, S.; Mohamadi, M.; Sarrafzadeh, M.-H.; Rene, E.R.; Firoozbahr, M. Recent advances in the treatment of dye-containing wastewater from textile industries: Overview and perspectives. Process Saf. Environ. 2020, 143, 138–163. [Google Scholar] [CrossRef]
- Lopez-Lopez, A.; Pic, J.S.; Debellefontaine, H. Ozonation of azo dye in a semi-batch reactor: A determination of the molecular and radical contributions. Chemosphere 2007, 66, 2120–2126. [Google Scholar] [CrossRef]
- Davies, L.C.; Cabrita, G.J.M.; Ferreira, R.A.; Carias, C.C.; Novais, J.M.; Martins-Dias, S. Integrated study of the role of Phragmites australis in azo-dye treatment in a constructed wetland: From pilot to molecular scale. Ecol. Eng. 2009, 35, 961–970. [Google Scholar] [CrossRef]
- Cai, Z.; Sun, Y.; Liu, W.; Pan, F.; Sun, P.; Fu, J. An overview of nanomaterials applied for removing dyes from wastewater. Environ. Sci. Pollut. Res. 2017, 24, 15882–15904. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jia, J.; Wang, Y. Enhanced photocatalytic-electrolytic degradation of Reactive Brilliant Red X-3B in the presence of water jet cavitation. Ultrason. Sonochem. 2015, 23, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Lei, X.; Li, X.; Ruan, Z.; Zhang, T.; Pan, F.; Li, Q.; Xia, D.; Fu, J. Adsorption-photocatalytic degradation of dye pollutant in water by graphite oxide grafted titanate nanotubes. J. Mol. Liq. 2018, 266, 122–131. [Google Scholar] [CrossRef]
- Latif, A.; Noor, S.; Sharif, Q.M.; Najeebullah, M. Different techniques recently used for the treatment of textile dyeing effluents: A review. J-Chem. Soc. Pak. 2010, 32, 115–124. [Google Scholar]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Lei, X.; You, M.; Pan, F.; Liu, M.; Yang, P.; Xia, D.; Li, Q.; Wang, Y.; Fu, J. CuFe2O4@GO nanocomposite as an effective and recoverable catalyst of peroxymonosulfate activation for degradation of aqueous dye pollutants. Chin. Chem. Lett. 2019, 30, 2216–2220. [Google Scholar] [CrossRef]
- Wang, N.; Zhu, L.; Wang, M.; Wang, D.; Tang, H. Sono-enhanced degradation of dye pollutants with the use of H2O2 activated by Fe3O4 magnetic nanoparticles as peroxidase mimetic. Ultrason. Sonochem. 2010, 17, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.K.; Dash, R.R.; Bhunia, P. A review on chemical coagulation/flocculation technologies for removal of colour from textile wastewaters. J. Environ. Manag. 2012, 93, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, J. Adsorption of C.I. Reactive Red 228 dye from aqueous solution by modified cellulose from flax shive: Kinetics, equilibrium, and thermodynamics. Ind. Crop. Prod. 2013, 42, 153–158. [Google Scholar] [CrossRef]
- Ramírez, G.; Recio, F.J.; Herrasti, P.; Ponce-de-León, C.; Sirés, I. Effect of RVC porosity on the performance of PbO2 composite coatings with titanate nanotubes for the electrochemical oxidation of azo dyes. Electrochim. Acta 2016, 204, 9–17. [Google Scholar] [CrossRef] [Green Version]
- Barragán, B.E.; Costa, C.; Carmen Márquez, M. Biodegradation of azo dyes by bacteria inoculated on solid media. Dyes Pigments 2007, 75, 73–81. [Google Scholar] [CrossRef]
- Paździor, K.; Bilińska, L.; Ledakowicz, S. A review of the existing and emerging technologies in the combination of AOPs and biological processes in industrial textile wastewater treatment. Chem. Eng. J. 2019, 376, 120597. [Google Scholar] [CrossRef]
- Soon, A.N.; Hameed, B.H. Degradation of Acid Blue 29 in visible light radiation using iron modified mesoporous silica as heterogeneous Photo-Fenton catalyst. Appl. Catal. A Gen. 2013, 450, 96–105. [Google Scholar] [CrossRef]
- Ortega-Liébana, M.C.; Sánchez-López, E.; Hidalgo-Carrillo, J.; Marinas, A.; Marinas, J.M.; Urbano, F.J. A comparative study of photocatalytic degradation of 3-chloropyridine under UV and solar light by homogeneous (photo-Fenton) and heterogeneous (TiO2) photocatalysis. Appl. Catal. B 2012, 127, 316–322. [Google Scholar] [CrossRef]
- Silva, A.M.T.; Herney-Ramirez, J.; Söylemez, U.; Madeira, L.M. A lumped kinetic model based on the Fermi’s equation applied to the catalytic wet hydrogen peroxide oxidation of Acid Orange 7. Appl. Catal. B 2012, 121–122, 10–19. [Google Scholar] [CrossRef]
- Maroudas, A.; Pandis, P.K.; Chatzopoulou, A.; Davellas, L.-R.; Sourkouni, G.; Argirusis, C. Synergetic decolorization of azo dyes using ultrasounds, photocatalysis and photo-fenton reaction. Ultrason. Sonochem. 2021, 71, 105367. [Google Scholar] [CrossRef]
- Perez, M.; Torrades, F.; Domenech, X. Fenton and photo-Fenton oxidation of textile effluents. Water Res. 2002, 36, 2703–2710. [Google Scholar] [CrossRef]
- Torrades, F.; García-Montaño, J. Using central composite experimental design to optimize the degradation of real dye wastewater by Fenton and photo-Fenton reactions. Dyes Pigments 2014, 100, 184–189. [Google Scholar] [CrossRef] [Green Version]
- Rios-Enriquez, M.; Shahin, N.; Durán-de-Bazúa, C.; Lang, J.; Oliveros, E.; Bossmann, S.H.; Braun, A.M. Optimization of the heterogeneous Fenton-oxidation of the model pollutant 2,4-xylidine using the optimal experimental design methodology. Sol. Energy 2004, 77, 491–501. [Google Scholar] [CrossRef]
- Li, Y.; Sun, J.; Sun, S.P. Mn2+-mediated homogeneous Fenton-like reaction of Fe(III)-NTA complex for efficient degradation of organic contaminants under neutral conditions. J. Hazard. Mater. 2016, 313, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, Y.; Xu, J. Separation of hydrogen sulfide from gas phase using Ce3+/Mn2+-enhanced fenton-like oxidation system. Chem. Eng. J. 2019, 359, 1486–1492. [Google Scholar] [CrossRef]
- Pope, F.D.; Hansen, J.C.; Bayes, K.D.; Friedl, R.R.; Sander, S.P. Ultraviolet absorption spectrum of chlorine peroxide, ClOOCl. J. Phys. Chem. A 2007, 111, 4322–4332. [Google Scholar] [CrossRef]
- Plapinger, R.E. Ultraviolet Absorption Spectra of Some Hydroxamic Acids and Hydroxamic Acid Derivatives. J. Org. Chem. 1959, 24, 802–804. [Google Scholar] [CrossRef]
- Li, Y.; Lu, Y.; Zhu, X. Photo-Fenton discoloration of the azo dye X-3B over pillared bentonites containing iron. J. Hazard. Mater. 2006, 132, 196–201. [Google Scholar] [CrossRef]
- Abdo, M.S.E.; Shaban, H.; Bader, M.S.H. Decolorization by ozone of direct dyes in presence of some catalysts. J. Environ. Sci. Health Part A Environ. Sci. Eng. 2008, 23, 697–710. [Google Scholar] [CrossRef]
- Jun, M.A.; Graham, N.J.D. Degradation of atrazine by manganese-catalysed ozonation: Influence of humic substances. Water Res. 1999, 33, 785–793. [Google Scholar]
- Fu, J.; Chen, G.; Yang, Y.; Zhang, Z.-M.; Zeng, Q.-F.; An, S.-Q.; Zhu, H.-L. Ultraviolet irradiation combined with manganese ore catalyzed ozonation of 4-chlorophenol in aqueous solution. Water Supply 2010, 10, 97–104. [Google Scholar] [CrossRef]
- Fu, J.; Zhang, Z.-M.; Tang, J.-Y.; Zeng, Q.-F.; An, S.-Q.; Zhu, H.-L. Photoreduction of Reactive Brilliant Red X-3B by Ultraviolet Irradiation/Potassium Borohydride/Sodium Bisulfite. J. Environ. Eng. 2010, 136, 1314–1319. [Google Scholar] [CrossRef]
- AlHamedi, F.H.; Rauf, M.A.; Ashraf, S.S. Degradation studies of Rhodamine B in the presence of UV/H2O2. Desalination 2009, 239, 159–166. [Google Scholar] [CrossRef]
- Watts, R.J.; Sarasa, J.; Loge, F.J.; Teel, A.L. Oxidative and Reductive Pathways in Manganese-Catalyzed Fenton’s Reactions. J. Environ. Eng. 2005, 131, 158–164. [Google Scholar] [CrossRef]
- Vellanki, B.P.; Batchelor, B.; Abdel-Wahab, A. Advanced Reduction Processes: A New Class of Treatment Processes. Environ. Eng. Sci. 2013, 30, 264–271. [Google Scholar] [CrossRef] [Green Version]
- Rauf, M.A.; Meetani, M.A.; Hisaindee, S. An overview on the photocatalytic degradation of azo dyes in the presence of TiO2 doped with selective transition metals. Desalination 2011, 276, 13–27. [Google Scholar] [CrossRef]
- Ghorai, K.; Panda, A.; Bhattacharjee, M.; Mandal, D.; Hossain, A.; Bera, P.; Seikh, M.M.; Gayen, A. Facile synthesis of CuCr2O4/CeO2 nanocomposite: A new Fenton like catalyst with domestic LED light assisted improved photocatalytic activity for the degradation of RhB, MB and MO dyes. Appl. Surf. Sci. 2021, 536, 147604. [Google Scholar] [CrossRef]
- Nikhila, M.P.; John, D.; Pai, M.R.; Renuka, N.K. Cu and Ag modified mesoporous TiO2 nanocuboids for visible light driven photocatalysis. Nano-Struct. Nano-Objects 2020, 21, 100420. [Google Scholar] [CrossRef]
- Lin, Z.R.; Zhao, L.; Dong, Y.H. Quantitative characterization of hydroxyl radical generation in a goethite-catalyzed Fenton-like reaction. Chemosphere 2015, 141, 7–12. [Google Scholar] [CrossRef]
- Xu, L.; Wang, J. Magnetic nanoscaled Fe3O4/CeO2 composite as an efficient Fenton-like heterogeneous catalyst for degradation of 4-chlorophenol. Environ. Sci. Technol. 2017, 46, 10145–10153. [Google Scholar] [CrossRef]
- Hwang, S.; Huling, S.G.; Ko, S. Fenton-like degradation of MTBE: Effects of iron counter anion and radical scavengers. Chemosphere 2010, 78, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Monteagudo, J.M.; Durán, A.; San Martin, I.; Carnicer, A. Roles of different intermediate active species in the mineralization reactions of phenolic pollutants under a UV-A/C photo-Fenton process. Appl. Catal. B 2011, 11, 152. [Google Scholar] [CrossRef]
- Fu, J.; Kyzas, G.Z.; Cai, Z.; Deliyanni, E.A.; Liu, W.; Zhao, D. Photocatalytic degradation of phenanthrene by graphite oxide-TiO2-Sr(OH)2/SrCO3 nanocomposite under solar irradiation: Effects of water quality parameters and predictive modeling. Chem. Eng. J. 2018, 335, 290–300. [Google Scholar] [CrossRef]
- Li, M.; Qiang, Z.; Pulgarin, C.; Kiwi, J. Accelerated methylene blue (MB) degradation by Fenton reagent exposed to UV or VUV/UV light in an innovative micro photo-reactor. Appl. Catal. B 2016, 187, 83–89. [Google Scholar] [CrossRef] [Green Version]
- Pirsaheb, M.; Moradi, S.; Shahlaei, M.; Wang, X.; Farhadian, N. Simultaneously implement of both weak magnetic field and aeration for ciprofloxacin removal by Fenton-like reaction. J. Environ. Manag. 2019, 246, 776–784. [Google Scholar] [CrossRef]
- Yang, X.; Warren, R.; He, Y.; Ye, J.; Li, Q.; Wang, G. Impacts of climate change on TN load and its control in a River Basin with complex pollution sources. Sci. Total Environ. 2018, 615, 1155–1163. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Graham, N.J.D.; Wang, W.; Liu, M.; Yu, W. Evaluating and Improving the Reliability of the UV-Persulfate Method for the Determination of TOC/DOC in Surface Waters. Water Res. 2021, 196, 116918. [Google Scholar] [CrossRef]
- Tee, H.C.; Lim, P.E.; Seng, C.E.; Mohd Nawi, M.A.; Adnan, R. Enhancement of azo dye Acid Orange 7 removal in newly developed horizontal subsurface-flow constructed wetland. J. Environ. Manag. 2015, 147, 349–355. [Google Scholar] [CrossRef]
- Ruan, X.-C.; Liu, M.-Y.; Zeng, Q.-F.; Ding, Y.-H. Degradation and decolorization of reactive red X-3B aqueous solution by ozone integrated with internal micro-electrolysis. Sep. Purif. Technol. 2010, 74, 195–201. [Google Scholar] [CrossRef]
- Wu, Y.; Qin, L.; Zhang, G.; Chen, L.; Guo, X.; Liu, M. Porous Solid Superacid SO42–/Fe2–xZrxO3 Fenton Catalyst for Highly Effective Oxidation of X-3B under Visible Light. Ind. Eng. Chem. Res. 2013, 52, 16698–16708. [Google Scholar] [CrossRef]
- Hua, L.; Ma, H.; Zhang, L. Degradation process analysis of the azo dyes by catalytic wet air oxidation with catalyst CuO/γ-Al2O3. Chemosphere 2013, 90, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Q.; Hu, X.M.; Chen, X.; Yang, J. The Study on the Reactive Brilliant Red X-3B Treatment with Internal Electrolysis-Fenton Oxidizing Method. Ind. Saf. Environ. Prot. 2010, 36, 34–35. [Google Scholar]














Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, G.; Wan, J.; Li, Q.; Sun, L.; Zhang, Y.; Li, Z.; Dang, C.; Fu, J. Degradation of Reactive Brilliant Red X-3B by Photo-Fenton-like Process: Effects of Water Chemistry Factors and Degradation Mechanism. Water 2022, 14, 380. https://doi.org/10.3390/w14030380
Cheng G, Wan J, Li Q, Sun L, Zhang Y, Li Z, Dang C, Fu J. Degradation of Reactive Brilliant Red X-3B by Photo-Fenton-like Process: Effects of Water Chemistry Factors and Degradation Mechanism. Water. 2022; 14(3):380. https://doi.org/10.3390/w14030380
Chicago/Turabian StyleCheng, Gong, Jing Wan, Qin Li, Lei Sun, Yibo Zhang, Zhang Li, Chenyuan Dang, and Jie Fu. 2022. "Degradation of Reactive Brilliant Red X-3B by Photo-Fenton-like Process: Effects of Water Chemistry Factors and Degradation Mechanism" Water 14, no. 3: 380. https://doi.org/10.3390/w14030380