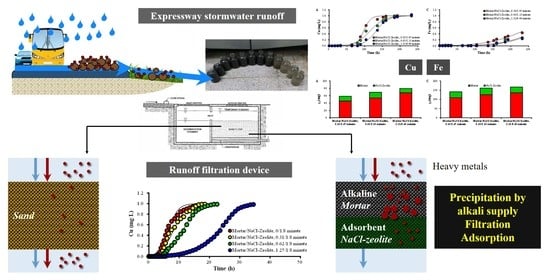
A Dual Media Filter using Zeolite and Mortar for the Efficient Removal of Heavy Metals in Stormwater Runoff
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Continuous Experiments
2.2.1. Removal of Cu2+ by Various Types of Filter Media
2.2.2. Removal of Cu2+ in Mortar/Na-Zeolite Dual Filter Media
2.2.3. Removal of Heavy Metals Mixture by Mortar/Na-Zeolite Dual Media
3. Results
3.1. Effects of Filter Media on Cu2+ Removal
3.2. Effects of Mortar Amount on the Cu2+ Removal in Dual Media
3.3. Removal of Heavy Metals Mixture by Mortar/Na-Zeolite Dual Media
3.3.1. Removal of Heavy Metals Mixture in Mortar Layer
3.3.2. Removal of Heavy Metals Mixture in the Entire Layers of Mortar/Na-Zeolite
3.3.3. Contribution of Each Layer in Mortar/Na-Zeolite Dual Media
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, D.-G.; Ko, S.-O. Road-Deposited Sediments Mediating the Transfer of Anthropogenic Organic Matter to Stormwater Runoff. Environ. Geochem. Health 2021, 43, 3287–3301. [Google Scholar] [CrossRef] [PubMed]
- Baum, P.; Kuch, B.; Dittmer, U. Adsorption of Metals to Particles in Urban Stormwater Runoff—Does Size Really Matter? Water 2021, 13, 309. [Google Scholar] [CrossRef]
- Wijeyawardana, P.; Nanayakkara, N.; Gunasekara, C.; Karunarathna, A.; Law, D.; Pramanik, B.K. Removal of Cu, Pb and Zn from Stormwater Using an Industrially Manufactured Sawdust and Paddy Husk Derived Biochar. Environ. Technol. Innov. 2022, 28, 102640. [Google Scholar] [CrossRef]
- Hwang, C.-C.; Weng, C.-H. Effects of Rainfall Patterns on Highway Runoff Pollution and Its Control. Water Environ. J. 2015, 29, 214–220. [Google Scholar] [CrossRef]
- Kayhanian, M.; Singh, A.; Suverkropp, C.; Borroum, S. Impact of Annual Average Daily Traffic on Highway Runoff Pollutant Concentrations. J. Environ. Eng. 2003, 129, 975–990. [Google Scholar] [CrossRef] [Green Version]
- Kayhanian, M.; Fruchtman, B.D.; Gulliver, J.S.; Montanaro, C.; Ranieri, E.; Wuertz, S. Review of Highway Runoff Characteristics: Comparative Analysis and Universal Implications. Water Res. 2012, 46, 6609–6624. [Google Scholar] [CrossRef]
- United States Department of Transportation (USDOT) and Federal Highway Administration (FHWA). Eisenhower Interstate Highway System Website. Available online: http://www.Fhwa.Dot.Gov/Interstate/Homepage.Cfm (accessed on 20 June 2017).
- Eriksson, E.; Baun, A.; Scholes, L.; Ledin, A.; Ahlman, S.; Revitt, M.; Noutsopoulos, C.; Mikkelsen, P.S. Selected Stormwater Priority Pollutants—A European Perspective. Sci. Total Environ. 2007, 383, 41–51. [Google Scholar] [CrossRef]
- Lee, J.; Lee, M. Stormwater Runoff Treatment Filtration System and Backwashing System. Water Sci. Technol. 2019, 79, 771–778. [Google Scholar] [CrossRef]
- Sharma, R.; Vymazal, J.; Malaviya, P. Application of Floating Treatment Wetlands for Stormwater Runoff: A Critical Review of the Recent Developments with Emphasis on Heavy Metals and Nutrient Removal. Sci. Total Environ. 2021, 777, 146044. [Google Scholar] [CrossRef]
- Korea Office of Prime Minister; Ministry for Food, Agriculture, Forestry and Fisheries; Korea Ministry of Knowledge Economy; Korea Ministry of Environment; Korea Ministry of Infrastructure and Transport; Korea Nation Fire Agency; Korea Rural Development Administration; Korea Forest Service. The Third Comprehensive Non-Point Sources Management Plan (2021−2025); Korea Ministry of Environment: Sejong, Korea, 2020. (In Korean)
- Bardin, J.P.; Gautier, A.; Barraud, S.; Chocat, B. The Purification Performance of Infiltration Basins Fitted with Pretreatment Facilities: A Case Study. Water Sci. Technol. 2001, 43, 119–128. [Google Scholar] [CrossRef]
- Hossain, M.A.; Furumai, H.; Nakajima, F.; Aryal, R.K. Heavy Metals Speciation in Soakaways Sediment and Evaluation of Metal Retention Properties of Surrounding Soil. Water Sci. Technol. 2007, 56, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, J.; Dierkes, C.; Göbel, P.; Klinger, C.; Stubbe, H.; Coldewey, W.G. Metal Concentrations in Soil and Seepage Water Due to Infiltration of Roof Runoff by Long Term Numerical Modelling. Water Sci. Technol. 2005, 51, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Barrett, M.; Katz, L.; Taylor, S. Removal of Dissolved Heavy Metals in Highway Runoff. Transp. Res. Rec. J. Transp. Res. Board 2014, 2436, 131–138. [Google Scholar] [CrossRef]
- Na Nagara, V.; Sarkar, D.; Elzinga, E.J.; Datta, R. Removal of Heavy Metals from Stormwater Runoff Using Granulated Drinking Water Treatment Residuals. Environ. Technol. Innov. 2022, 28, 102636. [Google Scholar] [CrossRef]
- Hsieh, C.-H.; Davis, A.P. Evaluation and Optimization of Bioretention Media for Treatment of Urban Storm Water Runoff. J. Environ. Eng. 2005, 131, 1521–1531. [Google Scholar] [CrossRef] [Green Version]
- Davis, A.P.; Shokouhian, M.; Sharma, H.; Minami, C.; Winogradoff, D. Water Quality Improvement through Bioretention: Lead, Copper, and Zinc Removal. Water Environ. Res. 2003, 75, 73–82. [Google Scholar] [CrossRef]
- Pitcher, S.K.; Slade, R.C.T.; Ward, N.I. Heavy Metal Removal from Motorway Stormwater Using Zeolites. Sci. Total Environ. 2004, 334–335, 161–166. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, P.; Yang, L.; Huang, T. Adsorption Characteristics of Construction Waste for Heavy Metals from Urban Stormwater Runoff. Chin. J. Chem. Eng. 2015, 23, 1542–1550. [Google Scholar] [CrossRef]
- Huber, M.; Hilbig, H.; Badenberg, S.C.; Fassnacht, J.; Drewes, J.E.; Helmreich, B. Heavy Metal Removal Mechanisms of Sorptive Filter Materials for Road Runoff Treatment and Remobilization under De-Icing Salt Applications. Water Res. 2016, 102, 453–463. [Google Scholar] [CrossRef]
- Ernst, C.; Katz, L.; Barrett, M. Removal of Dissolved Copper and Zinc from Highway Runoff via Adsorption. J. Sustain. Water Built. Environ. 2016, 2, 04015007. [Google Scholar] [CrossRef]
- Reddy, K.R.; Xie, T.; Dastgheibi, S. Removal of Heavy Metals from Urban Stormwater Runoff Using Different Filter Materials. J. Environ. Chem. Eng. 2014, 2, 282–292. [Google Scholar] [CrossRef]
- Günay, A.; Arslankaya, E.; Tosun, İ. Lead Removal from Aqueous Solution by Natural and Pretreated Clinoptilolite: Adsorption Equilibrium and Kinetics. J. Hazard. Mater. 2007, 146, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, C.R.; Rubio, J. New Basis for Adsorption of Ionic Pollutants onto Modified Zeolites. Miner. Eng. 2007, 20, 552–558. [Google Scholar] [CrossRef]
- Lee, M.; Saunders, J.A. Effects of PH on Metals Precipitation and Sorption: Field Bioremediation and Geochemical Modeling Approaches. Vadose Zone J. 2003, 2, 177–185. [Google Scholar] [CrossRef] [Green Version]
- Taffarel, S.R.; Rubio, J. On the Removal of Mn2+ Ions by Adsorption onto Natural and Activated Chilean Zeolites. Miner. Eng. 2009, 22, 336–343. [Google Scholar] [CrossRef]
- Todeschini, S.; Papiri, S.; Ciaponi, C. Performance of stormwater detention tanks for urban drainage systems in northern Italy. J. Environ. Manag. 2012, 101, 33–45. [Google Scholar] [CrossRef]
- Kayhanian, M.; Rasa, E.; Vichare, A.; Leatherbarrow, J.E. Utility of Suspended Solid measurements for storm-water runoff treatment. J. Environ. Eng. 2008, 134, 712–721. [Google Scholar] [CrossRef]
- Korea Ministry of Environment. Installation and Operation Manual of Non-Point Pollution Reduction Facility; Korea Ministry of Environment: Sejong, Korea, 2020. (In Korean)
- Thomas, H.C. Heterogeneous Ion Exchange in a Flowing System. J. Am. Chem. Soc. 1944, 66, 1664–1666. [Google Scholar] [CrossRef]
- Khalfa, L.; Sdiri, A.; Bagane, M.; Cervera, M.L. A Calcined Clay Fixed Bed Adsorption Studies for the Removal of Heavy Metals from Aqueous Solutions. J. Clean. Prod. 2021, 278, 123935. [Google Scholar] [CrossRef]
- Suksabye, P.; Thiravetyan, P.; Nakbanpote, W. Column study of chromium(VI) adsorption from electroplating industry by coconut coir pith. J. Hazard. Mater. 2008, 160, 56–62. [Google Scholar] [CrossRef]
- Sizirici, B.; Yildiz, I. Simultaneous removal of organics and metals in fixed bed using gravel and iron oxide coated gravel. Results Eng. 2020, 5, 100093. [Google Scholar] [CrossRef]
- Faisal, A.A.H.; Abdul-Kareem, M.B.; Mohammed, A.K.; Naushad, M.; Ghfar, A.A.; Ahamad, T. Humic acid coated sand as a novel sorbent in permeable reactive barrier for environmental remediation of groundwater polluted with copper and cadmium ions. J. Water Process Eng. 2020, 36, 101373. [Google Scholar] [CrossRef]
- Tasharrofi, S.; Rouzitalab, Z.; Maklavany, D.M.; Esmaeili, A.; Rabieezadeh, M.; Askarieh, M.; Rashidi, A.; Taghdisian, H. Adsorption of cadmium using modified zeolite-supported nanoscale zero-valent iron composites as a reactive material for PRBs. Sci. Total Environ. 2020, 736, 139570. [Google Scholar] [CrossRef] [PubMed]
- Winston, R.J.; Hunt, W.F. Characterizing Runoff from Roads: Particle Size Distributions, Nutrients, and Gross Solids. J. Environ. Eng. 2017, 143, 04016074. [Google Scholar] [CrossRef]
- Saadi, Z.; Saadi, R.; Fazaeli, R. Fixed-Bed Adsorption Dynamics of Pb (II) Adsorption from Aqueous Solution Using Nanostructured γ-Alumina. J. Nanostruct. Chem. 2013, 3, 48. [Google Scholar] [CrossRef] [Green Version]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions, 2nd ed.; Translated from the French by James A. Franklin (except Sections I, III 5 and III 6, Which Were Originally Written in English); National Association of Corrosion Engineers: Houston, TX, USA, 1974. [Google Scholar]
- Haile, T.M.; Fuerhacker, M. Simultaneous Adsorption of Heavy Metals from Roadway Stormwater Runoff Using Different Filter Media in Column Studies. Water 2018, 10, 1160. [Google Scholar] [CrossRef] [Green Version]
- Esfandiar, N.; Suri, R.; McKenzie, E.R. Competitive sorption of Cd, Cr, Cu, Ni, Pb and Zn from stormwater runoff by five low-cost sorbents; Effects of co-contaminants, humic acid, salinity and pH. J. Hazard. Mater. 2022, 423, 126938. [Google Scholar] [CrossRef]
- Sounthararajah, D.P.; Loganathan, P.; Kandasamy, J.; Vigneswaran, S. Removing heavy metals using permeable pavement system with a titanate nano-fibrous adsorbent column as a post treatment. Chemosphere 2017, 168, 467–473. [Google Scholar] [CrossRef]






| Condition | Sand | Na-Zeolite | Mortar/Na-Zeolite |
|---|---|---|---|
| Depth (cm) | 8.2 | 8.2 | 1 + 8.2 |
| EBCT (minute) | 1.8 | 1.8 | 0.2 + 1.8 |
| Mass (g) | 33.05 | 17.84 | 3.18 + 17.84 |
| Initial Cu2+ (mg/L) | 100 | 100 | 100 |
| Condition | Mortar/Na-Zeolite, 0/1.8 Min | Mortar/NaCl- Zeolite, 0.31/1.8 Min | Mortar/NaCl- Zeolite, 0.62/1.8 Min | Mortar/NaCl- Zeolite, 1.25/1.8 Min | |
|---|---|---|---|---|---|
| Depth (cm) | Mortar | 0 | 2 | 4 | 8 |
| Na-zeolite | 11 | 11 | 11 | 11 | |
| Total | 11 | 13 | 15 | 19 | |
| EBCT (minute) | Mortar | 0 | 0.3 | 0.6 | 1.3 |
| Na-zeolite | 1.8 | 1.8 | 1.8 | 1.8 | |
| Total | 1.8 | 2.1 | 2.4 | 3.1 | |
| Mass (g) | Mortar | 0 | 6.07 | 12.08 | 20.83 |
| Na-zeolite | 17.84 | 17.84 | 17.84 | 17.84 | |
| Total | 17.84 | 23.91 | 29.92 | 38.67 | |
| Condition | Mortar/Na-Zeolite, 0.33/1.47 Min | Mortar/Na-Zeolite, 0.65/1.15 Min | Mortar/Na-Zeolite, 1.31/0.49 Min | |
|---|---|---|---|---|
| Depth (cm) | Mortar | 2 | 4 | 8 |
| Na-zeolite | 9 | 7 | 3 | |
| Total | 11 | 11 | 11 | |
| EBCT (min) | Mortar | 0.33 | 0.65 | 1.31 |
| Na-zeolite | 1.47 | 1.15 | 0.49 | |
| Total | 1.80 | 1.80 | 1.80 | |
| Mass (g) | Mortar | 13.7 | 10.6 | 4.6 |
| Na-zeolite | 6.0 | 11.9 | 23.8 | |
| Total | 19.6 | 22.5 | 28.4 | |
| Sand | Na-Zeolite | Mortar/Na-Zeolite | |
|---|---|---|---|
| kT (×10−5 L/min·mg) | 278.6 | 11.7 | 8.5 |
| qe (mg/g) | 0.35 | 16.57 | 17.78 |
| r2 | 0.995 | 0.988 | 0.986 |
| qL (g/L) | 0.79 | 20.40 | 22.99 |
| Mortar/NaCl- Zeolite, 0/1.8 Min | Mortar/NaCl- Zeolite, 0.31/1.8 Min | Mortar/NaCl- Zeolite, 0.62/1.8 Min | Mortar/NaCl- Zeolite, 1.25/1.8 Min | |
|---|---|---|---|---|
| kT (L/min·mg) | 0.106 | 0.082 | 0.069 | 0.047 |
| qe (mg/g) | 22.0 | 20.5 | 22.7 | 35.6 |
| r2 | 0.986 | 0.984 | 0.998 | 0.998 |
| qt (mg) | 392.6 | 490.5 | 679.6 | 1378.1 |
| qL (g/L) | 27.1 | 28.8 | 34.8 | 56.2 |
| Mortar/Na-Zeolite, 0.33/1.47 Min | Mortar/Na-Zeolite, 0.65/1.15 Min | Mortar/Na-Zeolite, 1.31/0.49 Min | ||
|---|---|---|---|---|
| Cu2+ | kT (L/min·mg) | 0.589 | 0.689 | 0.680 |
| qe (mg/g) | 8.758 | 5.174 | 3.265 | |
| r2 | 0.974 | 0.940 | 0.956 | |
| qt (mg) | 45.5 | 53.8 | 67.9 | |
| qL (g/L) | 12.9 | 7.6 | 4.8 | |
| Zn2+ | kT (L/min·mg) | 3.505 | 2.599 | 5.141 |
| qe (mg/g) | 3.354 | 3.849 | 2.204 | |
| r2 | 0.997 | 0.997 | 0.998 | |
| qt (mg) | 17.4 | 40.0 | 45.8 | |
| qL (g/L) | 4.9 | 5.7 | 3.2 | |
| Fe3+ | kT (L/min·mg) | 0.267 | 0.220 | 0.192 |
| qe (mg/g) | 20.995 | 12.046 | 6.539 | |
| r2 | 0.961 | 0.942 | 0.905 | |
| qt (mg) | 109.2 | 125.3 | 136.0 | |
| qL (g/L) | 30.9 | 17.7 | 9.6 | |
| Ni2+ | kT (L/min·mg) | 1.225 | 0.885 | 2.013 |
| qe (mg/g) | 5.189 | 3.922 | 2.385 | |
| r2 | 0.995 | 0.984 | 0.994 | |
| qt (mg) | 27.0 | 40.8 | 49.6 | |
| qL (g/L) | 7.6 | 5.8 | 3.5 |
| Mortar/Na-Zeolite, 0.33/1.47 Min | Mortar/Na-Zeolite, 0.65/1.15 Min | Mortar/Na-Zeolite, 1.31/0.49 Min | ||
|---|---|---|---|---|
| Cu2+ | kT (L/min·mg) | 1.709 | 0.823 | 0.982 |
| qe (mg/g) | 3.461 | 3.556 | 3.224 | |
| r2 | 0.994 | 0.988 | 0.997 | |
| qt (mg) | 58.5 | 69.3 | 79.6 | |
| qL (g/L) | 3.0 | 3.6 | 4.1 | |
| Zn2+ | kT (L/min·mg) | 6.195 | 4.034 | 1.665 |
| qe (mg/g) | 2.837 | 2.538 | 2.311 | |
| r2 | 0.999 | 1.000 | 0.984 | |
| qt (mg) | 47.9 | 49.5 | 57.1 | |
| qL (g/L) | 2.5 | 2.5 | 2.9 | |
| Fe3+ | kT (L/min·mg) | 0.367 | 0.331 | 0.359 |
| qe (mg/g) | 8.287 | 8.365 | 6.775 | |
| r2 | 0.846 | 0.960 | 0.860 | |
| qt (mg) | 161.6 | 141.4 | 167.3 | |
| qL (g/L) | 8.3 | 7.3 | 8.6 | |
| Ni2+ | kT (L/min·mg) | 1.793 | 1.311 | 8.269 |
| qe (mg/g) | 2.750 | 2.594 | 2.114 | |
| r2 | 0.994 | 0.996 | 0.990 | |
| qt (mg) | 46.5 | 50.6 | 52.2 | |
| qL (g/L) | 2.4 | 2.6 | 2.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.-G.; Ko, S.-O. A Dual Media Filter using Zeolite and Mortar for the Efficient Removal of Heavy Metals in Stormwater Runoff. Water 2022, 14, 3567. https://doi.org/10.3390/w14213567
Kim D-G, Ko S-O. A Dual Media Filter using Zeolite and Mortar for the Efficient Removal of Heavy Metals in Stormwater Runoff. Water. 2022; 14(21):3567. https://doi.org/10.3390/w14213567
Chicago/Turabian StyleKim, Do-Gun, and Seok-Oh Ko. 2022. "A Dual Media Filter using Zeolite and Mortar for the Efficient Removal of Heavy Metals in Stormwater Runoff" Water 14, no. 21: 3567. https://doi.org/10.3390/w14213567