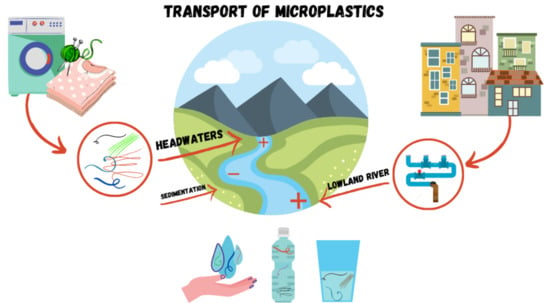
Microplastic Concentrations in Raw and Drinking Water in the Sinos River, Southern Brazil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Sampling Array
2.3. Extraction of Plastic Particles
2.4. Data Processing
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Aquatic Sciences and Fisheries Database (ASFA) ASFA. Available online: https://search-proquest.ez101.periodicos.capes.gov.br/asfaaquaculture/results/1BA41A9A5DEF4EA5PQ/1?accountid=26688 (accessed on 10 June 2020).
- Faure, F.; Corbaz, M.; Baecher, H.; Neuhaus, V.; Alencastro, L.F. Pollution due to Plastics and Microplastics in Lake Geneva. Arch. Sci. 2012, 65, 157–164. [Google Scholar]
- Castañeda, R.A.; Avlijas, S.; Simard, M.A.; Ricciardi, A. Microplastic pollution in St. Lawrence River sediments. Can. J. Fish. Aquat. Sci. 2014, 71, 1767–1771. [Google Scholar] [CrossRef]
- Brennecke, D.; Ferreira, E.C.; Costa, T.M.M.; Appel, D.; da Gama, B.A.P.; Lenz, M. Ingested microplastics (>100μm) are translocated to organs of the tropical fiddler crab Uca rapax. Mar. Pollut. Bull. 2015, 96, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Silva-Cavalcanti, J.S.; Silva, J.D.B.; de França, E.J.; de Araújo, M.C.B.; Gusmão, F. Microplastics ingestion by a common tropical freshwater fishing resource. Environ. Pollut. 2017, 221, 218–226. [Google Scholar] [CrossRef] [PubMed]
- McCormick, A.R.; Hoellein, T.J.; London, M.G.; Hittie, J.; Scott, J.W.; Kelly, J.J. Microplastic in surface waters of urban rivers: Concentration, sources, and associated bacterial assemblages. Ecosphere 2016, 7, e01556. [Google Scholar] [CrossRef]
- Nelms, S.E.; Galloway, T.S.; Godley, B.J.; Jarvis, D.S.; Lindeque, P.K. Investigating microplastic trophic transfer in marine top predators. Environ. Pollut. 2018, 238, 999–1007. [Google Scholar] [CrossRef]
- Tsang, Y.Y.; Mak, C.W.; Liebich, C.; Lam, S.W.; Sze, E.T.-P.; Chan, K.M. Microplastic pollution in the marine waters and sediments of Hong Kong. Mar. Pollut. Bull. 2017, 115, 20–28. [Google Scholar] [CrossRef]
- Ramírez-Álvarez, N.; Rios Mendoza, L.M.; Macías-Zamora, J.V.; Oregel-Vázquez, L.; Alvarez-Aguilar, A.; Hernández-Guzmán, F.A.; Sánchez-Osorio, J.L.; Moore, C.J.; Silva-Jiménez, H.; Navarro-Olache, L.F. Microplastics: Sources and distribution in surface waters and sediments of Todos Santos Bay, Mexico. Sci. Total Environ. 2020, 703, 134838. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.J.; Warrack, S.; Langen, V.; Challis, J.K.; Hanson, M.L.; Rennie, M.D. Microplastic contamination in Lake Winnipeg, Canada. Environ. Pollut. 2017, 225, 223–231. [Google Scholar] [CrossRef]
- Miller, R.Z.; Watts, A.J.R.; Winslow, B.O.; Galloway, T.S.; Barrows, A.P.W. Mountains to the sea: River study of plastic and non-plastic microfiber pollution in the northeast USA. Mar. Pollut. Bull. 2017, 124, 245–251. [Google Scholar] [CrossRef]
- Free, C.M.; Jensen, O.P.; Mason, S.A.; Eriksen, M.; Williamson, N.J.; Boldgiv, B. High-levels of microplastic pollution in a large, remote, mountain lake. Mar. Pollut. Bull. 2014, 85, 156–163. [Google Scholar] [CrossRef]
- Ravit, B.; Cooper, K.; Moreno, G.; Buckley, B.; Yang, I.; Deshpande, A.; Meola, S.; Jones, D.; Hsieh, A. Microplastics in urban New Jersey freshwaters: Distribution, chemical identification, and biological affects. AIMS Environ. Sci. 2017, 4, 809–826. [Google Scholar] [CrossRef]
- Tibbetts, J.; Krause, S.; Lynch, I.; Sambrook Smith, G.H. Abundance, Distribution and Drivers of Microplastic Contaminant in Urban River Environments. Water 2018, 10, 1597. [Google Scholar] [CrossRef] [Green Version]
- Castro, R.O.; da Silva, M.L.; de Araújo, F.V. Review on microplastic studies in Brazilian aquatic ecosystems. Ocean Coast. Manag. 2018, 165, 385–400. [Google Scholar] [CrossRef]
- Gomes, C.M.B. Lançamento de partículas de polietileno à costa do Rio Grande do Sul. Sep. Rev. Veritas 1973, 70/73, 174–206. [Google Scholar]
- De Carvalho, D.G.; Baptista Neto, J.A. Microplastic pollution of the beaches of Guanabara Bay, Southeast Brazil. Ocean Coast. Manag. 2016, 128, 10–17. [Google Scholar] [CrossRef]
- Olivatto, G.P.; Martins, M.C.T.; Montagner, C.C.; Henry, T.B.; Carreira, R.S. Microplastic contamination in surface waters in Guanabara Bay, Rio de Janeiro, Brazil. Mar. Pollut. Bull. 2019, 139, 157–162. [Google Scholar] [CrossRef]
- Vendel, A.L.; Bessa, F.; Alves, V.E.N.; Amorim, A.L.A.; Patrício, J.; Palma, A.R.T. Widespread microplastic ingestion by fish assemblages in tropical estuaries subjected to anthropogenic pressures. Mar. Pollut. Bull. 2017, 117, 448–455. [Google Scholar] [CrossRef]
- Andrade, M.C.; Winemiller, K.O.; Barbosa, P.S.; Fortunati, A.; Chelazzi, D.; Cincinelli, A.; Giarrizzo, T. First account of plastic pollution impacting freshwater fishes in the Amazon: Ingestion of plastic debris by piranhas and other serrasalmids with diverse feeding habits. Environ. Pollut. 2019, 244, 766–773. [Google Scholar] [CrossRef] [PubMed]
- Dris, R.; Gasperi, J.; Mirande, C.; Mandin, C.; Guerrouache, M.; Langlois, V.; Tassin, B. A first overview of textile fibers, including microplastics, in indoor and outdoor environments. Environ. Pollut. 2017, 221, 453–458. [Google Scholar] [CrossRef] [Green Version]
- Prata, J.C. Airborne microplastics: Consequences to human health? Environ. Pollut. 2018, 234, 115–126. [Google Scholar] [CrossRef]
- Waring, R.H.; Harris, R.M.; Mitchell, S.C. Plastic contamination of the food chain: A threat to human health? Maturitas 2018, 115, 64–68. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Mohamed Nor, N.H.; Hermsen, E.; Kooi, M.; Mintenig, S.M.; De France, J. Microplastics in freshwaters and drinking water: Critical review and assessment of data quality. Water Res. 2019, 155, 410–422. [Google Scholar] [CrossRef]
- Caracterização da Bacia Hidrográfica do Rio dos Sinos. Available online: http://www.comitesinos.com.br/bacia-hidrografica-do-rio-dos-sinos (accessed on 20 October 2020).
- Blume, K.; Macedo, J.; Meneguzzi, A.; Silva, L.; Quevedo, D.; Rodrigues, M. Water quality assessment of the Sinos River, Southern Brazil. Braz. J. Biol. 2010, 70, 1185–1193. [Google Scholar] [CrossRef] [Green Version]
- Karlsson, T.M.; Vethaak, A.D.; Almroth, B.C.; Ariese, F.; van Velzen, M.; Hassellöv, M.; Leslie, H.A. Screening for microplastics in sediment, water, marine invertebrates and fish: Method development and microplastic accumulation. Mar. Pollut. Bull. 2017, 122, 403–408. [Google Scholar] [CrossRef]
- Karami, A.; Golieskardi, A.; Choo, C.K.; Romano, N.; Ho, Y.B.; Salamatinia, B. A high-performance protocol for extraction of microplastics in fish. Sci. Total Environ. 2017, 578, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Maes, T.; Jessop, R.; Wellner, N.; Haupt, K.; Mayes, A.G. A rapid-screening approach to detect and quantify microplastics based on fluorescent tagging with Nile Red. Sci. Rep. 2017, 7, 44501. [Google Scholar] [CrossRef] [Green Version]
- Hendrickson, E.; Minor, E.C.; Schreiner, K. Microplastic Abundance and Composition in Western Lake Superior As Determined via Microscopy, Pyr-GC/MS, and FTIR. Environ. Sci. Technol. 2018, 52, 1787–1796. [Google Scholar] [CrossRef]
- Mintenig, S.M.; Löder, M.G.J.; Primpke, S.; Gerdts, G. Low numbers of microplastics detected in drinking water from ground water sources. Sci. Total Environ. 2019, 648, 631–635. [Google Scholar] [CrossRef]
- Di, M.; Wang, J. Microplastics in surface waters and sediments of the Three Gorges Reservoir, China. Sci. Total Environ. 2018, 616–617, 1620–1627. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 10 June 2020).
- Mani, T.; Hauk, A.; Walter, U.; Burkhardt-Holm, P. Microplastics profile along the Rhine River. Sci. Rep. 2016, 5, 17988. [Google Scholar] [CrossRef]
- Pivokonsky, M.; Cermakova, L.; Novotna, K.; Peer, P.; Cajthaml, T.; Janda, V. Occurrence of microplastics in raw and treated drinking water. Sci. Total Environ. 2018, 643, 1644–1651. [Google Scholar] [CrossRef]
- Vermaire, J.C.; Pomeroy, C.; Herczegh, S.M.; Haggart, O.; Murphy, M. Microplastic abundance and distribution in the open water and sediment of the Ottawa River, Canada, and its tributaries. FACETS 2017, 2, 301–314. [Google Scholar] [CrossRef] [Green Version]
- Pivokonský, M.; Pivokonská, L.; Novotná, K.; Čermáková, L.; Klimtová, M. Occurrence and fate of microplastics at two different drinking water treatment plants within a river catchment. Sci. Total Environ. 2020, 741, 140236. [Google Scholar] [CrossRef] [PubMed]
- Brossia, S. Corrosion of Pipes in Drinking Water Systems. In Handbook of Environmental Degradation of Materials; Elsevier: Amsterdam, The Netherlands, 2018; pp. 489–505. ISBN 978-0-323-52472-8. [Google Scholar]
- Zhao, S.; Zhu, L.; Wang, T.; Li, D. Suspended microplastics in the surface water of the Yangtze Estuary System, China: First observations on occurrence, distribution. Mar. Pollut. Bull. 2014, 86, 562–568. [Google Scholar] [CrossRef]
- Napper, I.E.; Thompson, R.C. Release of synthetic microplastic plastic fibres from domestic washing machines: Effects of fabric type and washing conditions. Mar. Pollut. Bull. 2016, 112, 39–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, M.F.; Ivar do Sul, J.A.; Silva-Cavalcanti, J.S.; Araújo, M.C.B.; Spengler, Â.; Tourinho, P.S. On the importance of size of plastic fragments and pellets on the strandline: A snapshot of a Brazilian beach. Environ. Monit. Assess. 2010, 168, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, L.K.; Bochow, M.; Imhof, H.K.; Oswald, S.E. Multi-temporal surveys for microplastic particles enabled by a novel and fast application of SWIR imaging spectroscopy—Study of an urban watercourse traversing the city of Berlin, Germany. Environ. Pollut. 2018, 239, 579–589. [Google Scholar] [CrossRef] [Green Version]
- Dris, R.; Gasperi, J.; Rocher, V.; Tassin, B. Synthetic and non-synthetic anthropogenic fibers in a river under the impact of Paris Megacity: Sampling methodological aspects and flux estimations. Sci. Total Environ. 2018, 618, 157–164. [Google Scholar] [CrossRef] [Green Version]
- Klein, S.; Worch, E.; Knepper, T.P. Occurrence and Spatial Distribution of Microplastics in River Shore Sediments of the Rhine-Main Area in Germany. Environ. Sci. Technol. 2015, 49, 6070–6076. [Google Scholar] [CrossRef]
- Yin, L.; Wen, X.; Du, C.; Jiang, J.; Wu, L.; Zhang, Y.; Hu, Z.; Hu, S.; Feng, Z.; Zhou, Z.; et al. Comparison of the abundance of microplastics between rural and urban areas: A case study from East Dongting Lake. Chemosphere 2020, 244, 125486. [Google Scholar] [CrossRef] [PubMed]
- Kosuth, M.; Mason, S.A.; Wattenberg, E.V. Anthropogenic contamination of tap water, beer, and sea salt. PLoS ONE 2018, 13, e0194970. [Google Scholar] [CrossRef] [PubMed]
- Dris, R.; Imhof, H.; Sanchez, W.; Gasperi, J.; Galgani, F.; Tassin, B.; Laforsch, C. Beyond the ocean: Contamination of freshwater ecosystems with (micro-)plastic particles. Environ. Chem. 2015, 12, 539–550. [Google Scholar] [CrossRef]
- Schwabl, P.; Köppel, S.; Königshofer, P.; Bucsics, T.; Trauner, M.; Reiberger, T.; Liebmann, B. Detection of Various Microplastics in Human Stool: A Prospective Case Series. Ann. Intern. Med. 2019, 171, 453–457. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, K.; Johnson, E.V.; Malmendal, A.; Linse, S.; Hansson, L.-A.; Cedervall, T. Brain damage and behavioural disorders in fish induced by plastic nanoparticles delivered through the food chain. Sci. Rep. 2017, 7, 11452. [Google Scholar] [CrossRef]
- World Health Organization. Microplastics in Drinking-Water; Licence: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2019; ISBN 978-92-4-151619-8. Available online: https://www.who.int/water_sanitation_health/publications/microplastics-in-drinking-water/en/ (accessed on 10 March 2020).
- Chen, Q.; Allgeier, A.; Yin, D.; Hollert, H. Leaching of endocrine disrupting chemicals from marine microplastics and mesoplastics under common life stress conditions. Environ. Int. 2019, 130, 104938. [Google Scholar] [CrossRef]
- Li, J.; Liu, H.; Paul Chen, J. Microplastics in freshwater systems: A review on occurrence, environmental effects, and methods for microplastics detection. Water Res. 2018, 137, 362–374. [Google Scholar] [CrossRef]
- Thompson, R.C.; Moore, C.J.; vom Saal, F.S.; Swan, S.H. Plastics, the environment and human health: Current consensus and future trends. Philos. Trans. Royal Soc. B Biol. Sci. 2009, 364, 2153–2166. [Google Scholar] [CrossRef]



| % Fibers | % Pellets | % Films | p | |
|---|---|---|---|---|
| Raw water | 89.4 | 10.5 | 0.1 | 0.996 |
| Drinking water | 80.2 | 19.1 | 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferraz, M.; Bauer, A.L.; Valiati, V.H.; Schulz, U.H. Microplastic Concentrations in Raw and Drinking Water in the Sinos River, Southern Brazil. Water 2020, 12, 3115. https://doi.org/10.3390/w12113115
Ferraz M, Bauer AL, Valiati VH, Schulz UH. Microplastic Concentrations in Raw and Drinking Water in the Sinos River, Southern Brazil. Water. 2020; 12(11):3115. https://doi.org/10.3390/w12113115
Chicago/Turabian StyleFerraz, Marlon, Amanda Leticia Bauer, Victor Hugo Valiati, and Uwe Horst Schulz. 2020. "Microplastic Concentrations in Raw and Drinking Water in the Sinos River, Southern Brazil" Water 12, no. 11: 3115. https://doi.org/10.3390/w12113115