Synthesis and Crystal Structure of Bis(2-phenylpyridine-C,N’)-bis(acetonitrile)iridium(III)hexafluorophosphate Showing Three Anion/Cation Couples in the Asymmetric Unit
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Crystallization
2.2. Structural Study
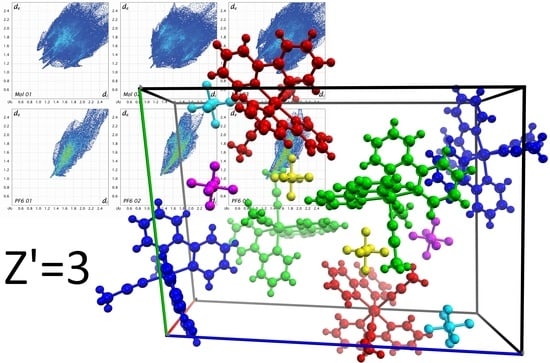
3. Results
4. Discussion
4.1. Structures with Z’ > 1 in the CCDC Database
4.2. Hirshfeld Surface Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nonoyama, M. Benzo[h]quinolin-10-yl-N Iridium(III) Complexes. Bull. Chem. Soc. Jpn. 1974, 47, 767–768. [Google Scholar] [CrossRef]
- King, K.A.; Spellane, P.J.; Watts, R.J. Excited-state properties of a triply ortho-metalated iridium(III) complex. J. Am. Chem. Soc. 1985, 107, 1431–1432. [Google Scholar] [CrossRef]
- Sprouse, S.; King, K.A.; Spellane, P.J.; Watts, R.J. Photophysical effects of metal-carbon. sigma. bonds in ortho-metalated complexes of iridium(III) and rhodium(III). J. Am. Chem. Soc. 1984, 106, 6647–6653. [Google Scholar] [CrossRef]
- Lamansky, S.; Djurovich, P.; Murphy, D.; Abdel-Razzaq, F.; Kwong, R.; Tsyba, I.; Bortz, M.; Mui, B.; Bau, R.; Thompson, M.E. Synthesis and Characterization of Phosphorescent Cyclometalated Iridium Complexes. Inorg. Chem. 2001, 40, 1704–1711. [Google Scholar] [CrossRef]
- Lamansky, S.; Djurovich, P.; Murphy, D.; Abdel-Razzaq, F.; Lee, H.-E.; Adachi, C.; Burrows, P.E.; Forrest, S.R.; Thompson, M.E. Highly Phosphorescent Bis-Cyclometalated Iridium Complexes: Synthesis, Photophysical Characterization, and Use in Organic Light Emitting Diodes. J. Am. Chem. Soc. 2001, 123, 4304–4312. [Google Scholar] [CrossRef]
- Gao, R.; Ho, D.G.; Hernandez, B.; Selke, M.; Murphy, D.; Djurovich, P.I.; Thompson, M.E. Bis-cyclometalated Ir(III) Complexes as Efficient Singlet Oxygen Sensitizers. J. Am. Chem. Soc. 2002, 124, 14828–14829. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-J.; Yang, C.-H.; Chen, K.; Chi, Y.; Shu, C.-F.; Ho, M.-L.; Yehc, Y.-S.; Chou, P.-T. Color tuning associated with heteroleptic cyclometalated Ir(III) complexes: Influence of the ancillary ligand. Dalt. Trans. 2007, 21, 1881–1890. [Google Scholar] [CrossRef] [PubMed]
- Volpi, G.; Garino, C.; Salassa, L.; Fiedler, J.; Hardcastle, K.I.; Gobetto, R.; Nervi, C. Cationic Heteroleptic Cyclometalated Iridium Complexes with 1-Pyridylimidazo[1,5-α]pyridine Ligands: Exploitation of an Efficient Intersystem Crossing. Chem. A Eur. J. 2009, 15, 6415–6427. [Google Scholar] [CrossRef] [PubMed]
- Housecroft, C.E.; Constable, E.C. Over the LEC rainbow: Colour and stability tuning of cyclometallated Iridium(III) complexes in light-emitting electrochemical cells. Coord. Chem. Rev. 2017, 350, 155–177. [Google Scholar] [CrossRef]
- Longhi, E.; De Cola, L. Iridium(III) Complexes for OLED Application. In Iridium(III) in Optoelectronic and Photonics Applications; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2017; pp. 205–274. [Google Scholar] [CrossRef]
- Pashaei, B.; Karimi, S.; Shahroosvand, H.; Abbasi, P.; Pilkington, M.; Bartolotta, A.; Fresta, E.; Fernandez-Cestau, J.; Costa, R.D.; Bonaccorso, F. Polypyridyl ligands as a versatile platform for solid-state light-emitting devices. Chem. Soc. Rev. 2019, 48, 5033–5139. [Google Scholar] [CrossRef]
- Lo, K.K.W.; Chung, C.K.; Lee, T.K.M.; Lui, L.H.; Tsang, K.H.K.; Zhu, N. New Luminescent Cyclometalated Iridium(III) Diimine Complexes as Biological Labeling Reagents. Inorg. Chem. 2003, 42, 6886–6897. [Google Scholar] [CrossRef] [PubMed]
- Flamigni, L.; Barbieri, A.; Sabatini, C.; Ventura, B.; Barigelletti, F. Photochemistry and Photophysics of Coordination Compounds: Iridium BT - Photochemistry and Photophysics of Coordination Compounds II. Top. Curr. Chem. 2007, 281, 143–203. [Google Scholar] [CrossRef]
- Campagna, S.; Putoriero, F.; Nastasi, F.; Bergamini, G.; Balzani, V. Photochemistry and Photophysics of Coordination Compounds: Ruthenium. Top. Curr. Chem. 2007, 280, 117–214. [Google Scholar]
- Costa, R.D.; Ortí, E.; Bolink, H.J.; Monti, F.; Accorsi, G.; Armaroli, N. Luminescent ionic transition-metal complexes for light-emitting electrochemical cells. Angew. Chem. Int. Ed. 2012, 51, 8178–8211. [Google Scholar] [CrossRef] [PubMed]
- Fresta, E.; Volpi, G.; Garino, C.; Barolo, C.; Costa, R.D. Contextualizing yellow light-emitting electrochemical cells based on a blue-emitting imidazo-pyridine emitter. Polyhedron 2018, 140, 129–137. [Google Scholar] [CrossRef]
- McGee, K.A.; Mann, K.R. Selective low-temperature syntheses of facial and meridional tris-cyclometalated iridium(III) complexes. Inorg. Chem. 2007, 46, 7800–7809. [Google Scholar] [CrossRef]
- Steed, J.W. Should solid-state molecular packing have to obey the rules of crystallographic symmetry? CrystEngComm 2003, 5, 169–179. [Google Scholar] [CrossRef]
- Steed, K.M.; Steed, J.W. Packing Problems: High Z’ Crystal Structures and Their Relationship to Cocrystals, Inclusion Compounds, and Polymorphism. Chem. Rev. 2015, 115, 2895–2933. [Google Scholar] [CrossRef]
- Lemmerer, A.; Fernandes, M.A. Adventures in co-crystal land: High Z’, stoichiometric variations, polymorphism and phase transitions in the co-crystals of four liquid and solid cyclic carboxylic acids with the supramolecular reagent isonicotinamide. New J. Chem. 2012, 36, 2242–2252. [Google Scholar] [CrossRef]
- Taylor, R.; Cole, J.C.; Groom, C.R. Molecular Interactions in Crystal Structures with Z’ > 1. Cryst. Growth Des. 2016, 16, 2988–3001. [Google Scholar] [CrossRef]
- Rajnikant; Gupta, V.K.; Kapoor, K.; Kumar, S.; Dhar, K.L. X-ray Structure Analysis Online Multiple Molecules in the Crystallographic Asymmetric Unit of (Z)-3-(3-Chlorophenyl)-2-phenyl acrylic acid. X-ray Struct. Anal. Online 2012, 28, 9–10. [Google Scholar]
- Ciborska, A.; Conterosito, E.; Milanesio, M.; Kazimierczuk, K.; Rzymowska, K.; Brzozowski, K.; Dołęga, A. The Syntheses and Crystal Structures of the First Disiloxane-1,3-dithiol and Its Cadmium Complex. Eur. J. Inorg. Chem. 2015, 2015, 3059–3065. [Google Scholar] [CrossRef]
- Conterosito, E.; Magistris, C.; Barolo, C.; Croce, G.; Milanesio, M. Synthesis, characterization and crystal structure of 6-Chloro-4,4′-dimethyl-2,2′-bipyridine and 4,4′-Dimethyl 2,2′-bipyridine N-Oxide. J. Mol. Struct. 2016, 1107, 337–343. [Google Scholar] [CrossRef]
- Toson, V.; Milanesio, M.; Conterosito, E. Crystal packing and layered morphology relationships in naphthalene sulfonate compounds. Zeitschrift für Kristallographie—Crystalline Materials 2017, 232, 463–469. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting intermolecular interactions in molecular crystals. CrystEngComm 2002, 4, 378. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem. Commun. 2007, 37, 3814–3816. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Spackman, M.A.; Mitchell, A.S. Novel Tools for Visualizing and Exploring Intermolecular Interactions in Molecular Crystals. Acta Crystallogr. B 2004, 60, 627–668. [Google Scholar] [CrossRef]
- Tankov, I.; Yankova, R. Mechanistic investigation of molecular geometry, intermolecular interactions and spectroscopic properties of pyridinium nitrate. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 219, 53–67. [Google Scholar] [CrossRef]
- Tankov, I.; Yankova, R. Hirshfeld surface, DFT vibrational (FT-IR) and electronic (UV–vis) studies on 4-amino-1H-1,2,4-triazolium nitrate. J. Mol. Struct. 2019, 1179, 581–592. [Google Scholar] [CrossRef]
- Rigaku Oxford Diffraction, CrysAlisPro Software System, version 1.171.38.46; Rigaku Corporation: Wroclaw, Poland, 2015.
- Burla, M.C.; Caliandro, R.; Carrozzini, B.; Cascarano, G.L.; Cuocci, C.; Giacovazzo, C.; Mallamo, M.; Mazzone, A.; Polidori, G. Crystal structure determination and refinement via SIR2014. J. Appl. Crystallogr. 2015, 48, 306–309. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Wolff, S.K.; Grimwood, D.J.; McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer; University of Western Australia: Crawley, Australia, 2017. [Google Scholar]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Cryst. 2006, 39, 453–457. [Google Scholar] [CrossRef] [Green Version]
- Leone, L.; Esteban-Gómez, D.; Platas-Iglesias, C.; Milanesio, M.; Tei, L. Accelerating water exchange in Gd III –DO3A-derivatives by favouring the dissociative mechanism through hydrogen bonding. Chem. Commun. 2019, 55, 513–516. [Google Scholar] [CrossRef] [PubMed]
- Capillas, C.; Tasci, E.S.; de la Flor, G.; Orobengoa, D.; Perez-Mato, J.M.; Aroyo, M.I. A new computer tool at the Bilbao Crystallographic Server to detect and characterize pseudosymmetry. Zeitschrift für Kristallographie—Crystalline Materials 2011, 226, 186–196. [Google Scholar] [CrossRef]
- Conifer, C.M.; Taylor, R.A.; Law, D.J.; Sunley, G.J.; White, A.J.P.; Britovsek, G.J.P. First metal complexes of 6,6′-dihydroxy-2,2′-bipyridine: From molecular wires to applications in carbonylation catalysis. Dalt. Trans. 2011, 40, 1031–1033. [Google Scholar] [CrossRef] [Green Version]
- Sun, B.; Guan, J.-X.; Xu, L.; Yu, B.-L.; Jiang, L.; Kou, J.-F.; Wang, L.; Ding, X.-D.; Chao, H.; Ji, L.-N. DNA Condensation Induced by Ruthenium(II) Polypyridyl Complexes [Ru(bpy) 2 (PIPSH)] 2+ and [Ru(bpy) 2 (PIPNH)] 2+. Inorg. Chem. 2009, 48, 4637–4639. [Google Scholar] [CrossRef]
- Akine, S.; Nagumo, H.; Nabeshima, T. Hierarchical Helix of Helix in the Crystal: Formation of Variable-Pitch Helical π-Stacked Array of Single-Helical Dinuclear Metal Complexes. Inorg. Chem. 2012, 51, 5506–5508. [Google Scholar] [CrossRef]
- Wah, H.L.K.; Postel, M.; Tomi, F.; Mordenti, L.; Ballivet-Tkatchenko, D.; Dahan, F.; Urso, F. Reaction of chlorobisnitrosyliron dimer with bidentate nitrogen ligands (2,2′-bipyridine, 4,4′-dimethyl 2,2′-bipyridine and 1,10-phenanthroline) X-ray structure of [Fe(bpy)3][Fe(NO)2Cl2]2. New J. Chem. 1991, 15, 629. [Google Scholar]
- Laws, K.; Eskandari, A.; Lu, C.; Suntharalingam, K. Highly Charged, Cytotoxic, Cyclometalated Iridium(III) Complexes as Cancer Stem Cell Mitochondriotropics. Chem. A Eur. J. 2018, 24, 15205–15210. [Google Scholar] [CrossRef]
- Zheng, Y.; Harms, K.; Zhang, L.; Meggers, E. Enantioselective Alkynylation of 2-Trifluoroacetyl Imidazoles Catalyzed by Bis-Cyclometalated Rhodium(III) Complexes Containing Pinene-Derived Ligands. Chem. A Eur. J. 2016, 22, 11977–11981. [Google Scholar] [CrossRef] [PubMed]
- Heeg, M.J.; Kroener, R.; Deutsch, E. IUCr Structure of cis-bis(acetonitrile)bis(2,2’-bipyridine)ruthenium(II) hexafluorophosphate, [Ru(C2H3N)2(C10H8N2)2](PF6)2. Acta Cryst. 1985, C41, 684–686. [Google Scholar] [CrossRef]
- Xu, F.; Huang, W. IUCr Redetermination of cis-diacetonitrilebis(2,2′-bipyridine)ruthenium(II) hexafluorophosphate. Acta Cryst. 2007, E63, m2114. [Google Scholar] [CrossRef]









| Unit Cell Parameters | Lengths of the Cell Edges (Å) | Angles (Degree) |
|---|---|---|
| Low temperature (172 K) | a 8.870(1) b 18.131(3) c 25.655(4) | α 93.993(2)° β 96.938(2)° γ 93.926(2)° |
| Room temperature (293K) | a 8.8635(5) b 18.186(1) c 25.624(2) | α 93.582(5)° β 96.034(5)° γ 93.842(5)° |
| Moiety 1 | Atom 1 | Atom 2 | Moiety 2 | Contact Distance (Å) |
|---|---|---|---|---|
| Mol 1 | C30 | H32 | Mol 1 | 2.852 |
| Mol 1 | H23 | C19 | Mol 3 | 2.818 |
| Mol 1 | H23 | C20 | Mol 3 | 2.635 |
| Mol 1 | H80A | F10 | PF6 2 | 2.359 |
| Mol 1 | H22 | F5 | PF6 1 | 2.346 |
| Mol 1 | H78C | F6 | PF6 1 | 2.618 |
| Mol 1 | C77 | F9 | PF6 2 | 3.159 |
| Mol 1 | H34 | F12 | PF6 2 | 2.604 |
| Mol 1 | H37 | F12 | PF6 2 | 2.634 |
| Mol 1 | H40 | F9 | PF6 2 | 2.608 |
| Mol 2 | C57 | H49 | Mol 2 | 2.588 |
| Mol 2 | H58 | C81 | Mol 2 | 2.787 |
| Mol 2 | H82B | F2 | PF6 1 | 2.566 |
| Mol 2 | H82B | F3 | PF6 1 | 2.558 |
| Mol 2 | H58 | F11 | PF6 2 | 2.588 |
| Mol 2 | H59 | F16 | PF6 3 | 2.492 |
| Mol 2 | H82A | F17 | PF6 3 | 2.653 |
| Mol 3 | C19 | H23 | Mol 1 | 2.818 |
| Mol 3 | C20 | H23 | Mol 1 | 2.635 |
| Mol 3 | H18 | C10 | Mol 3 | 2.715 |
| Mol 3 | C20 | H76C | Mol 3 | 2.837 |
| Mol 3 | H76A | F1 | PF6 1 | 2.45 |
| Mol 3 | H74C | F3 | PF6 1 | 2.459 |
| Mol 3 | H1 | F1 | PF6 1 | 2.704 |
| Mol 3 | H1 | F4 | PF6 1 | 2.593 |
| Mol 3 | H76B | F15 | PF6 3 | 2.441 |
| Mol 2 | H12 | F18 | PF6 3 | 2.663 |
| Mol 3 | H11 | F18 | PF6 3 | 2.628 |
| Mol 3 | H14 | F20 | PF6 3 | 2.628 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fresta, E.; Milanesio, M.; Volpi, G.; Barolo, C.; Conterosito, E. Synthesis and Crystal Structure of Bis(2-phenylpyridine-C,N’)-bis(acetonitrile)iridium(III)hexafluorophosphate Showing Three Anion/Cation Couples in the Asymmetric Unit. Crystals 2019, 9, 617. https://doi.org/10.3390/cryst9120617
Fresta E, Milanesio M, Volpi G, Barolo C, Conterosito E. Synthesis and Crystal Structure of Bis(2-phenylpyridine-C,N’)-bis(acetonitrile)iridium(III)hexafluorophosphate Showing Three Anion/Cation Couples in the Asymmetric Unit. Crystals. 2019; 9(12):617. https://doi.org/10.3390/cryst9120617
Chicago/Turabian StyleFresta, Elisa, Marco Milanesio, Giorgio Volpi, Claudia Barolo, and Eleonora Conterosito. 2019. "Synthesis and Crystal Structure of Bis(2-phenylpyridine-C,N’)-bis(acetonitrile)iridium(III)hexafluorophosphate Showing Three Anion/Cation Couples in the Asymmetric Unit" Crystals 9, no. 12: 617. https://doi.org/10.3390/cryst9120617