Hydrogen Desorption Properties of Bulk and Nanoconfined LiBH4-NaAlH4
Abstract
:1. Introduction
2. Experimental Details
2.1. Sample Preparation
2.2. Sample Characterization
3. Results and Discussion
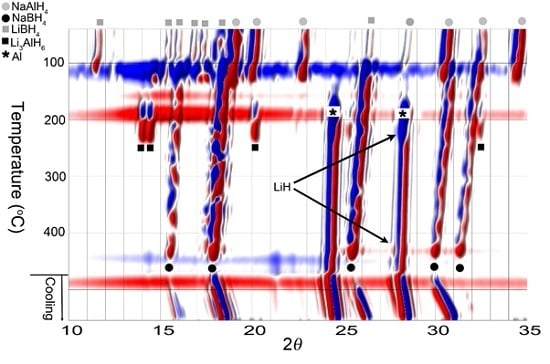
3.1. In Situ Synchrotron Radiation—Powder X-Ray Diffraction of Bulk and Nanoconfined 2LiBH4-NaAlH4
3.2. Nanoporous Carbon Aerogel Composite
3.3. Decomposition of Bulk and Nanocomposites of 2LiBH4-NaAlH4
3.4. Cyclic Stability
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ley, M.B.; Jepsen, L.H.; Lee, Y.S.; Cho, Y.W.; von Colbe, J.M.B.; Dornheim, M.; Rokni, M.; Jensen, J.O.; Sloth, M.; Filinchuk, Y.; et al. Complex hydrides for hydrogen storage—New perspectives. Mater. Today 2014, 17, 122–128. [Google Scholar] [CrossRef]
- Callini, E.; Atakli, Z.Ö.K.; Hauback, B.C.; Orimo, S.-I.; Jensen, C.; Dornheim, M.; Grant, D.; Cho, Y.W.; Chen, P.; Hjörvarsson, B.; et al. Complex and liquid hydrides for energy storage. Appl. Phys. A 2016, 122, 1–22. [Google Scholar] [CrossRef]
- Crivello, J.C.; Denys, R.V.; Dornheim, M.; Felderhoff, M.; Grant, D.M.; Huot, J.; Jensen, T.R.; de Jongh, P.; Latroche, M.; Walker, G.S.; et al. Mg-based compounds for hydrogen and energy storage. Appl. Phys. A 2016, 122, 1–17. [Google Scholar] [CrossRef]
- Crivello, J.C.; Dam, B.; Denys, R.V.; Dornheim, M.; Grant, D.M.; Huot, J.; Jensen, T.R.; de Jongh, P.; Latroche, M.; Milanese, C.; et al. Review of magnesium hydride-based materials: Development and optimisation. Appl. Phys. A 2016, 122, 1–20. [Google Scholar] [CrossRef]
- Yartys, V.; Noreus, D.; Latroche, M. Metal hydrides as negative electrode materials for Ni-MH batteries. Appl. Phys. A 2016, 122, 1–11. [Google Scholar] [CrossRef]
- Lototskyy, M.V.; Yartys, V.A.; Pollet, B.G.; Bowman, R.C. Metal hydride hydrogen compressors: A review. Int. J. Hydrog. Energy 2014, 39, 5818–5851. [Google Scholar] [CrossRef]
- Zuttel, A.; Wenger, P.; Rentsch, S.; Sudan, P.; Mauron, P.; Emmenegger, C. LiBH4 a new hydrogen storage material. J. Power Sour. 2003, 118, 1–7. [Google Scholar] [CrossRef]
- Yang, J.; Sudik, A.; Wolverton, C. Destabilizing LiBH4 with a metal (M = Mg, Al, Ti, V, Cr, or Sc) or metal hydride (MH2, MgH2, TiH2, or CaH2). J. Phys. Chem. C 2007, 111, 19134–19140. [Google Scholar] [CrossRef]
- Yu, X.B.; Grant, D.A.; Walker, G.S. Low-temperature dehydrogenation of LiBH4 through destabilization with TiO2. J. Phys. Chem. C 2008, 112, 11059–11062. [Google Scholar] [CrossRef]
- Meggouh, M.; Grant, D.M.; Walker, G.S. Optimizing the destabilization of LiBH4 for hydrogen storage and the effect of different Al sources. J. Phys. Chem. C 2011, 115, 22054–22061. [Google Scholar] [CrossRef]
- Purewal, J.; Hwang, S.J.; Bowman, R.C.; Ronnebro, E.; Fultz, B.; Ahn, C. Hydrogen sorption behavior of the ScH2-LiBH4 system: Experimental assesment of chemical destabilization effects. J. Phys. Chem. C 2008, 112, 8481–8485. [Google Scholar] [CrossRef]
- Ravnsbaek, D.B.; Jensen, T.R. Mechanism for reversible hydrogen storage in LiBH4-Al. J. Appl. Phys. 2012, 111, 112621. [Google Scholar] [CrossRef]
- Kang, X.D.; Wang, P.; Ma, L.P.; Cheng, H.M. Reversible hydrogen storage in LiBH4 destabilized by milling with Al. Appl. Phys. A 2007, 89, 963–966. [Google Scholar] [CrossRef]
- Hansen, B.R.S.; Ravnsbaek, D.B.; Reed, D.; Book, D.; Gundlach, C.; Skibsted, J.; Jensen, T.R. Hydrogen storage capacity loss in a LiBH4-Al composite. J. Phys. Chem. C 2013, 117, 7423–7432. [Google Scholar] [CrossRef]
- Gao, J.; Adelhelm, P.; Verkuijlen, M.H.W.; Rongeat, C.; Herrich, M.; van Bentum, P.J.M.; Gutfleisch, O.; Kentgens, A.P.M.; de Jong, K.P.; de Jongh, P.E. Confinement of NaAlH4 in nanoporous carbon: Impact on H2 release, reversibility, and thermodynamics. J. Phys. Chem. C 2010, 114, 4675–4682. [Google Scholar] [CrossRef]
- Bogdanovic, B.; Brand, R.A.; Marjanovic, A.; Schwickardi, M.; Tolle, J. Metal-doped sodium aluminium hydrides as potential new hydrogen storage materials. J. Alloys Compd. 2000, 302, 36–58. [Google Scholar] [CrossRef]
- Ravnsbaek, D.B.; Jensen, T.R. Tuning hydrogen storage properties and reactivity: Investigation of the LiBH4-NaAlH4 system. J. Phys. Chem. Solids 2010, 71, 1144–1149. [Google Scholar] [CrossRef]
- Shi, Q.; Yu, X.; Feidenhans’l, R.; Vegge, T. Destabilized LiBH4-NaAlH4 mixtures doped with titanium based catalysts. J. Phys. Chem. C 2008, 112, 18244–18248. [Google Scholar] [CrossRef]
- De Jongh, P.E.; Adelhelm, P. Nanosizing and nanoconfinement: New strategies towards meeting hydrogen storage goals. ChemSusChem 2010, 3, 1332–1348. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.K.; Besenbacher, F.; Jensen, T.R. Nanoconfined hydrides for energy storage. Nanoscale 2011, 3, 2086–2098. [Google Scholar] [CrossRef] [PubMed]
- Paskevicius, M.; Sheppard, D.A.; Buckley, C.E. Thermodynamic changes in mechanochemically synthesized magnesium hydride nanoparticles. J. Am. Chem. Soc. 2010, 132, 5077–5083. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.K.; Polanski, M.; Zasada, D.; Javadian, P.; Besenbacher, F.; Bystrzycki, J.; Skibsted, J.; Jensen, T.R. Improved hydrogen storage kinetics of nanoconfined NaAlH4 catalyzed with TiCl3 nanoparticles. ACS Nano 2011, 5, 4056–4064. [Google Scholar] [CrossRef] [PubMed]
- Javadian, P.; Nielsen, T.K.; Ravnsbaek, D.B.; Jepsen, L.H.; Polanski, M.; Plocinski, T.; Kunce, I.; Besenbacher, F.; Bystrzycki, J.; Jensen, T.R. Scandium functionalized carbon aerogel: Synthesis of nanoparticles and structure of a new ScOCl and properties of NaAlH4 as a function of pore size. J. Solid State Chem. 2015, 231, 190–197. [Google Scholar] [CrossRef]
- Nielsen, T.K.; Javadian, P.; Polanski, M.; Besenbacher, F.; Bystrzycki, J.; Jensen, T.R. Nanoconfined NaALH4: Determination of distinct prolific effects from pore size, crystallite size, and surface interactions. J. Phys. Chem. C 2012, 116, 21046–21051. [Google Scholar] [CrossRef]
- Nielsen, T.K.; Bösenberg, U.; Gosalawit, R.; Dornheim, M.; Cerenius, Y.; Besenbacher, F.; Jensen, T.R. A reversible nanoconfined chemical reaction. ACS Nano 2010, 4, 3903–3908. [Google Scholar] [CrossRef] [PubMed]
- Al-Muhtaseb, S.A.; Ritter, J.A. Preparation and properties of resorcinol-formaldehyde organic and carbon gels. Adv. Mater. 2003, 15, 101–114. [Google Scholar] [CrossRef]
- Thiangviriya, S.; Plerdsranoy, P.; Wiset, N.; Javadian, P.; Jensen, T.R.; Utke, R. Hydrogen sorption and reaction mechanisms of nanoconfined 2LiBH4-NaAlH4. J Alloys Compd 2015, 633, 484–493. [Google Scholar] [CrossRef]
- Jensen, T.R.; Nielsen, T.K.; Filinchuk, Y.; Jorgensen, J.-E.; Cerenius, Y.; Gray, E.M.; Webb, C.J. Versatile in situ powder X-ray diffraction cells for solid-gas investigations. J. Appl. Crystallogr. 2010, 43, 1456–1463. [Google Scholar] [CrossRef] [PubMed]
- Hansen, B.R.S.; Moller, K.T.; Paskevicius, M.; Dippel, A.C.; Walter, P.; Webb, C.J.; Pistidda, C.; Bergemann, N.; Dornheim, M.; Klassen, T.; et al. In situ X-ray diffraction environments for high-pressure reactions. J. Appl. Crystallogr. 2015, 48, 1234–1241. [Google Scholar] [CrossRef]
- Paskevicius, M.; Ley, M.B.; Sheppard, D.A.; Jensen, T.R.; Buckley, C.E. Eutectic melting in metal borohydrides. Phys. Chem. Chem. Phys. 2013, 15, 19774–19789. [Google Scholar] [CrossRef] [PubMed]
- Stasinevich, D.; Egorenko, G.; Gnedina, G. Tiermograficzieskoie Issliedovanie Systiemy Gidridoborat Natrija-Gidrid Natrija. Dokl. Akad. Nauk SSSR 1966, 168, 610–612. [Google Scholar]
- Dilts, J.A.; Ashby, E.C. Study of thermal-decomposition of complex metal hydrides. Inorg. Chem. 1972, 11, 1230. [Google Scholar] [CrossRef]
- Javadian, P.; Jensen, T.R. Enhanced hydrogen reversibility of nanoconfined LiBH4-Mg(BH4)2. Int. J. Hydrog. Energy 2014, 39, 9871–9876. [Google Scholar] [CrossRef]
- Javadian, P.; Sheppard, D.A.; Buckley, C.E.; Jensen, T.R. Hydrogen storage properties of nanoconfined LiBH4-Ca(BH4)2. Nano Energy 2015, 11, 96–103. [Google Scholar] [CrossRef]
- Javadian, P.; Sheppard, D.A.; Buckley, C.E.; Jensen, T.R. Hydrogen storage properties of nanoconfined LiBH4-NaBH4. Int. J. Hydrog. Energy 2015, 40, 14916–14924. [Google Scholar] [CrossRef]
- Ward, P.A.; Teprovich, J.A.; Peters, B.; Wheeler, J.; Compton, R.N.; Zidan, R. Reversible hydrogen storage in a LiBH4-C60 nanocomposite. J. Phys. Chem. C 2013, 117, 22569–22575. [Google Scholar] [CrossRef]
- Varin, R.A.; Zbroniec, Z. Decomposition behavior of unmilled and ball milled lithium alanate (LiAlH4) including long-term storage and moisture effects. J. Alloys Compd. 2010, 504, 89–101. [Google Scholar] [CrossRef]
- Pitt, M.P.; Paskevicius, M.; Brown, D.H.; Sheppard, D.A.; Buckley, C.E. Thermal stability of Li2B12H12 and its role in the decomposition of LiBH4. J. Am. Chem. Soc. 2013, 135, 6930–6941. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.F.; Guo, Z.P.; Nevirkovets, I.P.; Liu, H.K.; Dou, S.X. Hydrogen de-/absorption improvement of NaBH4 catalyzed by titanium-based additives. J. Phys. Chem. C 2012, 116, 1596–1604. [Google Scholar] [CrossRef]
- Fu, R.; Yoshizawa, N.; Dresselhaus, M.S.; Dresselhaus, G.; Satcher, J.H.; Baumann, T.F. XPS Study of Copper-Doped Carbon Aerogels. Langmuir 2002, 18, 10100–10104. [Google Scholar] [CrossRef]






| Scaffold | SBET (m2/g) | Vmicro (mL/g) | Vmeso (mL/g) | Vtot (mL/g) | Dmax (nm) | LiNa * (wt. %) | Theoretical H2 Content (wt. %)# |
|---|---|---|---|---|---|---|---|
| CA | 689 ± 26 | 0.21 ± 0.02 | 1.06 ± 0.10 | 1.21 ±0.10 | 30 ± 0.15 | - | - |
| CA-LiNa | 257 ± 26 | 0.06 ± 0.02 | 0.71 ± 0.10 | 0.78 ±0.10 | 21 ± 0.15 | 25.3 | 2.6 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Javadian, P.; Sheppard, D.A.; Buckley, C.E.; Jensen, T.R. Hydrogen Desorption Properties of Bulk and Nanoconfined LiBH4-NaAlH4. Crystals 2016, 6, 70. https://doi.org/10.3390/cryst6060070
Javadian P, Sheppard DA, Buckley CE, Jensen TR. Hydrogen Desorption Properties of Bulk and Nanoconfined LiBH4-NaAlH4. Crystals. 2016; 6(6):70. https://doi.org/10.3390/cryst6060070
Chicago/Turabian StyleJavadian, Payam, Drew A. Sheppard, Craig E. Buckley, and Torben R. Jensen. 2016. "Hydrogen Desorption Properties of Bulk and Nanoconfined LiBH4-NaAlH4" Crystals 6, no. 6: 70. https://doi.org/10.3390/cryst6060070