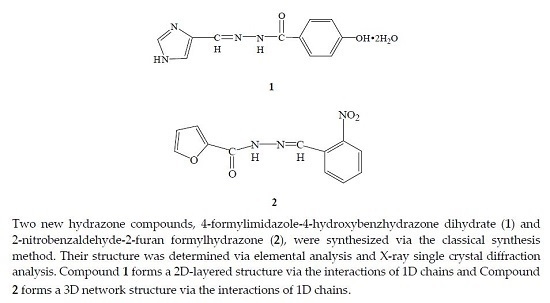
The Synthesis and Crystal Structure of Two New Hydrazone Compounds
Abstract
:1. Introduction
2. Results and Discussion
Description of Compounds 1 and 2
3. Experimental Section
3.1. Materials and Instrumentation
3.2. Preparation of Two Hydrazone Compounds
3.3. Crystal Structure Determination
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yang, Y.G.; Hong, M.; Xu, L.D.; Cui, J.C.; Chang, G.L.; Li, D.C.; Li, C.Z. Organotin(IV) complexes derived from Schiff base N′-[(1E)-(2-hydroxy-3-methoxyphenyl)methylidene]pyridine-3-carbohydrazone: Synthesis, in vitro cytotoxicities and DNA/BSA interaction. J. Organomet. Chem. 2016, 804, 48–58. [Google Scholar] [CrossRef]
- Mihkel, I.; Kristjan, T.; Anastasia, S.; Silver, T.; Uno, M. Synthesis of Novel Saccharide Hydrazones. Synth. Commun. 2015, 45, 1367–1373. [Google Scholar]
- Tai, X.S.; Feng, Y.M. 2-Hydroxy-N′-[1-(3-methylpyrazin-2-yl)-ethylidene]benzohydrazide. Acta Crystallogr. Sect. E 2008, 64, o707. [Google Scholar] [CrossRef] [PubMed]
- Evranos-Aksöz, B.; Onurdağ, F.K.; Özgacar, S.Ö. Antibacterial, antifungal and antimycobaterial activities of some pyrazoline, hydrazone and derivaties. Z. Naturforsch. C 2015, 70, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Jayanthi, E.; Anusuya, M.; Bhuvanesh, N.S.P.; Khalil, K.A.; Dharmaraj, N. Synthesis and characterization of ruthenium(II) hydrazone complexes as anticancer chemotherapeutic agents: In vitro DNA/BSA protein binding and cytotoxicity assay. J. Coord. Chem. 2015, 68, 3551–3565. [Google Scholar] [CrossRef]
- Rodríguez-Hermida, S.; Lago, A.B.; Carballo, R.; Fabelo, O.; Vázquez-López, E.M. Homo- and heteronuclear compounds with a symmetrical bis-hydrazone ligand: Synthesis, structural studies, and luminescent properties. Chemistry 2015, 21, 6605–6616. [Google Scholar] [CrossRef] [PubMed]
- Anacona, J.R.; Rangel, V.; Loroño, M.; Camus, J. Tetradentate metal complexes derived from cephalexin and 2,6-diacetylpyridine bis(hydrazone): Synthesis, characterization and antibacterial activity. Spectrochim. Acta A 2015, 149, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Raj, V.; Kumar, P.; Kumar, A. Design and synthesis of 4-(4-ethyl-phenyl)-4-oxo-butyric acid (3-oxo-1,3-diphenyl-propylidene)/[1-(4-chloro-phenyl)-ethylidene]-hydrazide as potential anticonvulsant agents. Med. Chem. Res. 2015, 24, 603–610. [Google Scholar] [CrossRef]
- Hunoor, R.S.; Patil, B.R.; Badiger, D.S.; Chandrashekhar, V.M.; Muchchandi, I.S.; Gudasi, K.B. Co(II), Ni(II), Cu(II) and Zn(II) complexes of isatinyl-2-aminobenzoylhydrazone: Synthesis, characterization and anticancer activity. Appl. Organomet. Chem. 2015, 29, 101–108. [Google Scholar] [CrossRef]
- Souza, L.G.S.; Almeida, M.C.S.; Lemos, T.L.G.; Ribeiro, P.R.V.; Brito, E.S.D.; Silva, V.L.M.; Silva, A.M.S.; Braz-Filho, R.; Costa, J.G.M.; Rodrigues, F.F.G.; et al. Synthesis, antibacterial and cytotoxic activities of new biflorin-based hydrazones and oximes. Bioorg. Med. Chem. Lett. 2016, 26, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Moraes, R.S.; Aderne, R.E.; Cremona, M.; Rey, N.A. Luminescent properties of a di-hydrazone derived from the antituberculosis agent isoniazid: Potentiality as an emitting layer constituent for OLED fabrication. Opt. Mater. 2016, 52, 186–191. [Google Scholar] [CrossRef]
- Tai, X.S.; Wang, H.Q.; Sun, X.Z.; Tan, M.Y. Synthesis and spectral characterization of methyl-2-pyridyl ketone benzoyl hydrazone and its complexes with rare earth nitrates. Spectrosc. Lett. 2005, 38, 497–504. [Google Scholar] [CrossRef]
- Tai, X.S.; Yin, J.; Kong, F.Y. Crystal structure of 2-carboxybenzaldehyde furan-2-carbohydrazide methanol hemisolvate. Z. Kristallogr. NCS 2007, 222, 401–402. [Google Scholar]
- Tai, X.S.; Yin, X.H.; Tan, M.Y.; Li, Y.Z. 3-Indolylformaldehyde isonicotinoylhydrazone methanol solvate. Acta Crystallogr. Sect. E 2003, 59, o681–o682. [Google Scholar] [CrossRef]
- Tai, X.S.; Wang, G.L.; Liu, Y.Y. Synthesis and crystal structure of a Na(I) complex with 4,4′-bipyridine and 2-formyl-benzenesulfonate-hydrazine. Sci. Study Res. Chem. Chem. Eng. Biotechnol. Food Ind. 2015, 16, 173–177. [Google Scholar]
- Tai, X.S.; Wang, X. Synthesis and crystal structure of a 1D-chained coordination polymer constructed from Ca2+ and 2-[(E)-(2-furoylhydrazono)methyl]benzenesulfonate. Crystals 2015, 5, 458–465. [Google Scholar] [CrossRef]
- Lei, Y.; Li, T.Z.; Fu, C.; Guan, X.L.; Tan, R. Synthesis and crystal structures of 4-chloro-N′-(4-hydroxy-3-nitrobenzylidene)benzohydrazide monohydrate and 4-chloro-N′-(4-hydroxy-3-methoxybenzylidene)benzohydrazide monohydrate. J. Chem. Crystallogr. 2011, 41, 1707–1711. [Google Scholar] [CrossRef]
- Zhang, Q.F.; Pang, J.D.; Zeng, S.Y.; Liu, C.H.; Sun, D.Z. Synthesis, characterization and crystal structure of N,N′-di[(E)-1-(2-hydoxyphenyl)methylidene]-2,6-naphthalenedicarbohydrazide. J. Chem. Crystallogr. 2012, 42, 271–275. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXL-97, Program for Crystal Structure Solution; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Sheldrick, G.M. SHELXTL-97, Program for Crystal Structure Refinement; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]




| Formula | C11H14N4O4 | C12H9N3O4 |
|---|---|---|
| Formula weight | 266.26 | 259.22 |
| Crystal system | triclinic | monoclinic |
| Space group | P-1 | P21/c |
| a [Å] | 7.0321(14) | 17.3618(9) |
| b [Å] | 7.3723(15) | 9.1506(4) |
| c [Å] | 13.008(3) | 15.5801(7) |
| α [°] | 98.66(3) | 90.00 |
| β [°] | 101.69(3) | 104.532(5) |
| γ [°] | 92.25(3) | 90.00 |
| Z | 2 | 8 |
| F(000) | 280 | 1072 |
| Temperature [K] | 293(2) | 293(2) |
| V [Å3] | 651.2(2) | 2396.05(19) |
| Calculated density [g·cm−3] | 1.358 | 1.437 |
| Crystal size [mm3] | 0.21×0.20×0.19 | 0.21×0.20×0.19 |
| μ [mm−1] | 0.106 | 0.111 |
| S | 1.046 | 1.028 |
| Limiting indices | −8 ≤ h ≤ 9, | −20 ≤ h ≤ 14, |
| −9 ≤ k ≤ 9, | −6 ≤ k ≤ 10, | |
| −16 ≤ l ≤ 16 | −15 ≤ l ≤ 18 | |
| Reflections collected | 6352 | 9815 |
| Unique reflections | 2949 | 4221 |
| Parameters | 172 | 344 |
| Restraints | 5 | 0 |
| Rint | 0.031 | 0.0477 |
| R1, wR2 [all data] | 0.0655, 0.1497 | 0.0970, 0.1925 |
| R1, wR2 [I>2σ(I)] | 0.0564, 0.1420 | 0.0633, 0.1649 |
| Largest diff.peak and hole [e·Å−3] | 0.412, −0.302 | 0.422, −0.321 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.-H.; Tai, X.-S. The Synthesis and Crystal Structure of Two New Hydrazone Compounds. Crystals 2016, 6, 57. https://doi.org/10.3390/cryst6050057
Wang L-H, Tai X-S. The Synthesis and Crystal Structure of Two New Hydrazone Compounds. Crystals. 2016; 6(5):57. https://doi.org/10.3390/cryst6050057
Chicago/Turabian StyleWang, Li-Hua, and Xi-Shi Tai. 2016. "The Synthesis and Crystal Structure of Two New Hydrazone Compounds" Crystals 6, no. 5: 57. https://doi.org/10.3390/cryst6050057