Surface Groups and Dielectric Properties of Ti3C2Tx MXene Nanosheets after NH3·H2O Solvothermal Treatment under Different Temperatures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Ti3C2Tx MXene
2.3. Solvothermal Synthesis of Ti3C2Tx MXene Nanosheet (MNS)
2.4. Characterization
3. Results and Discussion
3.1. Preparation of Ti3C2Tx MXene
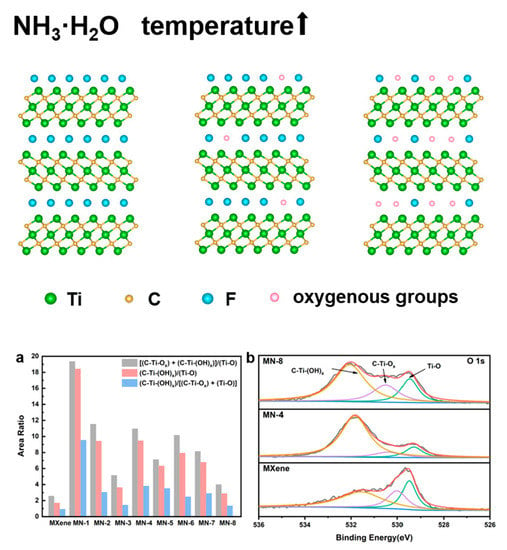
3.2. Solvothermal Treatment
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kumar, A.; Yaduvanshi, R.S. Nano Spherical Dielectric Resonator Antenna for Rectenna Application. Wirel. Pers. Commun. 2023, 128, 161–172. [Google Scholar] [CrossRef]
- Su, T.; Ma, X.; Tong, J.; Ji, H.; Qin, Z.; Wu, Z. Surface engineering of MXenes for energy and environmental applications. J. Mater. Chem. A 2022, 10, 10265–10296. [Google Scholar] [CrossRef]
- Xu, X.; Chen, J.; Li, B. Phonon thermal conduction in novel 2D materials. J. Phys. Condens. Matter 2016, 28, 483001. [Google Scholar] [CrossRef] [Green Version]
- Fu, Z.; Wang, N.; Legut, D.; Si, C.; Zhang, Q.; Du, S.; Germann, T.C.; Francisco, J.S.; Zhang, R. Rational Design of Flexible Two-Dimensional MXenes with Multiple Functionalities. Chem. Rev. 2019, 119, 11980–12031. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Bao, W.; Jaumaux, P.; Zhang, S.; Wang, C.; Wang, G. MXene-Based Composites: Synthesis and Applications in Rechargeable Batteries and Supercapacitors. Adv. Mater. Interfaces 2019, 6, 1802004. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Z.; Lu, W.; Hou, B. Theoretical Study of Electromagnetic Interference Shielding of 2D MXenes Films. Metals 2018, 8, 652. [Google Scholar] [CrossRef]
- Lin, L.; Sherrell, P.; Liu, Y.; Lei, W.; Zhang, S.; Zhang, H.; Wallace, G.G.; Chen, J. Engineered 2D Transition Metal Dichalcogenides—A Vision of Viable Hydrogen Evolution Reaction Catalysis. Adv. Energy Mater 2020, 10, 1903870. [Google Scholar] [CrossRef]
- Wang, Q.; O’Hare, D. Recent Advances in the Synthesis and Application of Layered Double Hydroxide (LDH) Nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef]
- Khan, U.; May, P.; O’Neill, A.; Bell, A.P.; Boussac, E.; Martin, A.; Semple, J.; Coleman, J.N. Polymer reinforcement using liquid-exfoliated boron nitride nanosheets. Nanoscale 2013, 5, 581–587. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.; Song, H.; Lin, S.; Zhou, Y.; Zhan, X.; Hu, Z.; Zhang, Q.; Sun, J.; Yang, B.; Li, T.; et al. Scalable salt-templated synthesis of two-dimensional transition metal oxides. Nat. Commun. 2016, 7, 11296. [Google Scholar] [CrossRef] [Green Version]
- Xiao, X.; Yu, H.; Jin, H.; Wu, M.; Fang, Y.; Sun, J.; Hu, Z.; Li, T.; Wu, J.; Huang, L.; et al. Salt-Templated Synthesis of 2D Metallic MoN and Other Nitrides. ACS Nano 2017, 11, 2180–2186. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, F.; Iqbal, A.; Kim, H.; Koo, C.M. 2D Transition Metal Carbides (MXenes): Applications as an Electrically Conducting Material. Adv. Mater. 2020, 32, 2002159. [Google Scholar] [CrossRef] [PubMed]
- Lukatskaya, M.R.; Mashtalir, O.; Ren, C.E.; Dall’Agnese, Y.; Rozier, P.; Taberna, P.L.; Naguib, M.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. Cation intercalation and high volumetric capacitance of two-dimensional titanium carbide. Science 2013, 341, 1502–1505. [Google Scholar] [CrossRef] [Green Version]
- VahidMohammadi, A.; Rosen, J.; Gogotsi, Y. The world of two-dimensional carbides and nitrides (MXenes). Science 2021, 372, 6547. [Google Scholar] [CrossRef] [PubMed]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.; Gogotsi, Y.; Barsoum, M.W. Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature 2014, 516, 78–81. [Google Scholar] [CrossRef]
- Kamysbayev, V.; Filatov, A.S.; Hu, H.; Xue, R.; Lagunas, F.; Wang, D.; Klie, R.F.; Talapin, D.V. Covalent surface modifications and superconductivity of two-dimensional metal carbide MXenes. Science 2020, 369, 979–983. [Google Scholar] [CrossRef]
- Lim, K.R.G.; Handoko, A.D.; Nemani, S.K.; Wyatt, B.; Jiang, H.; Tang, J.; Anasori, B.; Seh, Z.W. Rational Design of Two-Dimensional Transition Metal Carbide/Nitride (MXene) Hybrids and Nanocomposites for Catalytic Energy Storage and Conversion. ACS Nano 2020, 14, 10834–10864. [Google Scholar] [CrossRef]
- Tang, Q.; Zhou, Z.; Shen, P. Are MXenes Promising Anode Materials for Li Ion Batteries? Computational Studies on Electronic Properties and Li Storage Capability of Ti3C2 and Ti3C2X2(X = F, OH) Monolayer. J. Am. Chem. Soc. 2012, 134, 16909–16916. [Google Scholar] [CrossRef]
- Tu, S.; Jiang, Q.; Zhang, J.; He, X.; Hedhili, M.N.; Zhang, X.; Alshareef, H.N. Enhancement of Dielectric Permittivity of Ti3C2Tx MXene/Polymer Composites by Controlling Flake Size and Surface Termination. ACS Appl. Mater. Interfaces 2019, 11, 27358–27362. [Google Scholar] [CrossRef]
- Guo, J.; Peng, Q.; Fu, H.; Zou, G.; Zhang, Q. Heavy-Metal Adsorption Behavior of Two-Dimensional Alkalization-Intercalated MXene by First-Principles Calculations. J. Phys. Chem. C 2015, 119, 20923–20930. [Google Scholar] [CrossRef]
- Persson, I.; Halim, J.; Hansen, T.W.; Wagner, J.B.; Darakchieva, V.; Palisaitis, J.; Rosen, J.; Persson, P.O.Å. How Much Oxygen Can a MXene Surface Take Before It Breaks. Adv. Funct. Mater. 2020, 30, 1909005. [Google Scholar] [CrossRef] [Green Version]
- Yasui, K.; Kato, K. Influence of Adsorbate-Induced Charge Screening, Depolarization Factor, Mobile Carrier Concentration, and Defect-Induced Microstrain on the Size Effect of a BaTiO3 Nanoparticle. J. Phys. Chem. C 2013, 117, 19632–19644. [Google Scholar] [CrossRef]
- Andersen, K.; Latini, S.; Thygesen, K.S. Dielectric Genome of van der Waals Heterostructures. Nano Lett. 2015, 15, 4616–4621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, T.; Scullion, D.; Hughes, D.; Li, L.H.; Shih, C.-J.; Coleman, J.N.; Chhowalla, M.; Santos, E.J.G. Electronic Polarizability as the Fundamental Variable in the Dielectric Properties of Two- Dimensional Materials. Nano Lett. 2020, 20, 841–851. [Google Scholar] [CrossRef] [Green Version]







| Samples | Treating Temperature/°C | Elemental Proportion/% | Atom Ratio | ||||
|---|---|---|---|---|---|---|---|
| C | Ti | O | F | O/F | O/Ti | ||
| MXene | - | 40.22 | 24.77 | 17.61 | 17.40 | 1.01 | 0.71 |
| MN-1 | 40 | 39.44 | 13.80 | 36.82 | 9.95 | 3.70 | 2.67 |
| MN-2 | 60 | 36.97 | 14.17 | 42.34 | 6.52 | 6.49 | 2.99 |
| MN-3 | 80 | 41.08 | 16.77 | 35.94 | 6.21 | 5.79 | 2.14 |
| MN-4 | 100 | 47.33 | 9.14 | 39.53 | 4.01 | 9.86 | 4.32 |
| MN-5 | 120 | 38.19 | 15.87 | 39.2 | 6.74 | 5.82 | 2.47 |
| MN-6 | 140 | 36.69 | 14.95 | 43.55 | 4.81 | 9.05 | 2.91 |
| MN-7 | 160 | 41.71 | 12.56 | 42.13 | 3.60 | 11.70 | 3.35 |
| MN-8 | 180 | 39.15 | 19.50 | 36.16 | 5.19 | 6.97 | 1.85 |
| Samples | Treating Temperature/°C | Position/eV | Area Ratio (α) | ||||
|---|---|---|---|---|---|---|---|
| Ti-O | C-Ti-Ox | C-Ti-(OH)x | [(C-Ti-Ox) + (C-Ti-(OH)x)]/(Ti-O) | (C-Ti-(OH)x)/(Ti-O) | (C-Ti-(OH)x)/[(C-Ti-Ox) + (Ti-O)] | ||
| MXene | - | 529.55 | 530.30 | 531.80 | 2.57 | 1.71 | 0.92 |
| MN-1 | 40 | 529.12 | 530.15 | 531.66 | 19.35 | 18.42 | 9.53 |
| MN-2 | 60 | 529.26 | 530.15 | 531.82 | 11.53 | 9.43 | 3.05 |
| MN-3 | 80 | 529.19 | 530.15 | 531.70 | 5.14 | 3.64 | 1.45 |
| MN-4 | 100 | 529.12 | 530.20 | 531.79 | 10.96 | 9.48 | 3.82 |
| MN-5 | 120 | 529.30 | 530.40 | 531.83 | 7.12 | 6.33 | 3.53 |
| MN-6 | 140 | 529.10 | 530.20 | 531.72 | 10.16 | 7.94 | 2.47 |
| MN-7 | 160 | 529.14 | 530.25 | 531.72 | 8.14 | 6.79 | 2.89 |
| MN-8 | 180 | 529.47 | 530.50 | 532.04 | 4.00 | 2.87 | 1.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Li, G.; Zhao, Y.; Chen, X. Surface Groups and Dielectric Properties of Ti3C2Tx MXene Nanosheets after NH3·H2O Solvothermal Treatment under Different Temperatures. Crystals 2023, 13, 1005. https://doi.org/10.3390/cryst13071005
Liu Z, Li G, Zhao Y, Chen X. Surface Groups and Dielectric Properties of Ti3C2Tx MXene Nanosheets after NH3·H2O Solvothermal Treatment under Different Temperatures. Crystals. 2023; 13(7):1005. https://doi.org/10.3390/cryst13071005
Chicago/Turabian StyleLiu, Zhiwei, Guanlong Li, Yan Zhao, and Xiangbao Chen. 2023. "Surface Groups and Dielectric Properties of Ti3C2Tx MXene Nanosheets after NH3·H2O Solvothermal Treatment under Different Temperatures" Crystals 13, no. 7: 1005. https://doi.org/10.3390/cryst13071005