Review on Y6-Based Semiconductor Materials and Their Future Development via Machine Learning
Abstract
:1. Introduction
2. Basic Principle of Organic Solar Cells
3. Figures of Merit of Organic Solar Cells
3.1. Power Conversion Efficiency
3.2. Fill Factor
3.3. Short Circuit Current Density
3.4. Voltage Loss
4. Synthesis and Properties of Y6 Material
4.1. Material Design
4.2. Material Synthesis
4.3. Absorption Behaviors
4.4. Band Properties
5. Progress of the Improvement in Y6 Material
5.1. Fine-Tuning the Flanking Unit
5.2. Creating a New Core Moiety
5.3. Altering the Donor Units in A-DAD-A
5.4. Tuning the Alkyl Chain R1
5.5. Extending the Conjugation Length
5.6. Modulating the Side Chain R2
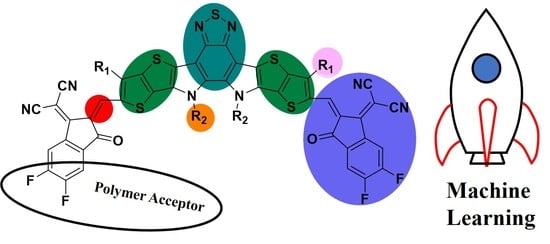
5.7. Polymer Acceptor
6. Future and Perspectives
6.1. Possibilities
6.2. Necessities
6.3. Future of Machine Learning in OSCs
6.3.1. Key Challenges
- (a)
- New and more Datasets
- (b)
- Suitable Fingerprints
6.3.2. Applications
- (a)
- Filtering before Synthesis
- (b)
- Predicting and even Unifying More Things Together
- (c)
- Aid Theories Behind
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gueymard, C.A. The sun’s total and spectral irradiance for solar energy applications and solar radiation models. Sol. Energy 2004, 76, 423–453. [Google Scholar] [CrossRef]
- Britt, J.; Ferekides, C. Thin-film CdS/CdTe solar cell with 15.8% efficiency. Appl. Phys. Lett. 1993, 62, 2851–2852. [Google Scholar] [CrossRef]
- Goetzberger, A.; Hebling, C.; Schock, H. Photovoltaic materials, history, status and outlook. Mater. Sci. Eng. R Rep. 2003, 40, 1–46. [Google Scholar] [CrossRef]
- Sun, C.; Xia, R.; Shi, H.; Yao, H.; Liu, X.; Hou, J.; Huang, F.; Yip, H.; Cao, Y. Heat-Insulating Multifunctional Semitransparent Polymer Solar Cells. Joule 2018, 2, 1816–1826. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Xia, R.; Zhang, G.; Yip, H.; Cao, Y. Spectral Engineering of Semitransparent Polymer Solar Cells for Greenhouse Applications. Adv. Energy Mater. 2019, 9, 1803438. [Google Scholar] [CrossRef]
- Han, Y.W.; Jeon, S.J.; Lee, H.S.; Park, H.; Kim, K.S.; Lee, H.W.; Moon, D.K. Evaporation-Free Nonfullerene Flexible Organic Solar Cell Modules Manufactured by An All-Solution Process. Adv. Energy Mater. 2019, 9, 1902065. [Google Scholar] [CrossRef]
- Dong, S.; Jia, T.; Zhang, K.; Jing, J.; Huang, F. Single-Component Non-halogen Solvent-Processed High-Performance Organic Solar Cell Module with Efficiency over 14%. Joule 2020, 4, 2004–2016. [Google Scholar] [CrossRef]
- Park, S.H.; Roy, A.; Beaupré, S.; Cho, S.; Coates, N.; Ji, S.M.; Moses, D.; Leclerc, M.; Lee, K.; Heeger, A.J. Bulk heterojunction solar cells with internal quantum efficiency approaching 100%. Nat. Photonics 2009, 3, 297–302. [Google Scholar] [CrossRef]
- Sariciftci, N.S.; Smilowitz, L.; Heeger, A.J.; Wudl, F. Photoinduced electron transfer from a conducting polymer to buckminsterfullerene. Science 1992, 258, 1474–1476. [Google Scholar] [CrossRef]
- Blom, P.W.M.; Mihailetchi, V.D.; Koster, L.J.A.; Markov, D.E. Device Physics of Polymer:Fullerene Bulk Heterojunction Solar Cells. Adv. Mater. 2007, 19, 1551–1566. [Google Scholar] [CrossRef] [Green Version]
- Dennler, G.; Scharber, M.C.; Brabec, C.J. Polymer-Fullerene Bulk-Heterojunction Solar Cells. Adv. Mater. 2009, 21, 1323–1338. [Google Scholar] [CrossRef]
- Reese, M.O.; Nardes, A.M.; Rupert, B.L.; Larsen, R.E.; Olson, D.C.; Lloyd, M.T.; Shaheen, S.E.; Ginley, D.S.; Rumbles, G.; Kopidakis, N. Photoinduced Degradation of Polymer and Polymer-Fullerene Active Layers: Experiment and Theory. Adv. Funct. Mater. 2010, 20, 3476–3483. [Google Scholar] [CrossRef]
- You, J.; Dou, L.; Yoshimura, K.; Kato, T.; Ohya, K.; Moriarty, T.; Emery, K.; Chen, C.; Gao, J.; Li, G.; et al. A polymer tandem solar cell with 10.6% power conversion efficiency. Nat. Commun. 2013, 4, 1446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Troisi, A. What Makes Fullerene Acceptors Special as Electron Acceptors in Organic Solar Cells and How to Replace Them. Adv. Mater. 2013, 25, 1038–1041. [Google Scholar] [CrossRef]
- Zhao, J.; Li, Y.; Yang, G.; Jiang, K.; Lin, H.; Ade, H.; Ma, W.; Yan, H. Efficient organic solar cells processed from hydrocarbon solvents. Nat. Energy 2016, 1, 15027. [Google Scholar] [CrossRef]
- Zhan, X.; Tan, Z.; Domercq, B.; An, Z.; Zhang, X.; Barlow, S.; Li, Y.; Zhu, D.; Kippelen, B.; Marder, S.R. A High-Mobility Electron-Transport Polymer with Broad Absorption and Its Use in Field-Effect Transistors and All-Polymer Solar Cells. J. Am. Chem. Soc. 2007, 129, 7246–7247. [Google Scholar] [CrossRef]
- Cheng, P.; Li, G.; Zhan, X.; Yang, Y. Next-generation organic photovoltaics based on non-fullerene acceptors. Nat. Photonics 2018, 12, 131–142. [Google Scholar] [CrossRef]
- Jørgensen, M.; Norrman, K.; Gevorgyan, S.A.; Tromholt, T.; Andreasen, B.; Krebs, F.C. Stability of Polymer Solar Cells. Adv. Mater. 2012, 24, 580–612. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, J.; Zhang, Z.; Bai, H.; Li, Y.; Zhu, D.; Zhan, X. An Electron Acceptor Challenging Fullerenes for Efficient Polymer Solar Cells. Adv. Mater. 2015, 27, 1170–1174. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Zhang, Y.; Zhou, L.; Zhang, G.; Yip, H.; Lau, T.; Lu, X.; Zhu, C.; Peng, H.; Johnson, P.A.; et al. Single-Junction Organic Solar Cell with over 15% Efficiency Using Fused-Ring Acceptor with Electron-Deficient Core. Joule 2019, 3, 1140–1151. [Google Scholar] [CrossRef]
- Cui, Y.; Yao, H.; Zhang, J.; Zhang, T.; Wang, Y.; Hong, L.; Xian, K.; Xu, B.; Zhang, S.; Peng, J.; et al. Over 16% efficiency organic photovoltaic cells enabled by a chlorinated acceptor with increased open-circuit voltages. Nat. Commun. 2019, 10, 2515. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhou, J.; Song, J.; Xu, J.; Zhang, H.; Zhang, X.; Guo, J.; Zhu, L.; Wei, D.; Han, G.; et al. Non-fullerene acceptors with branched side chains and improved molecular packing to exceed 18% efficiency in organic solar cells. Nat. Energy 2021, 6, 605–613. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, X.; Xiao, J.; Chow, P.C.Y.; Ren, M.; Kupgan, G.; Jiao, X.; Chan, C.C.S.; Du, X.; Xia, R.; et al. Delocalization of exciton and electron wavefunction in non-fullerene acceptor molecules enables efficient organic solar cells. Nat. Commun. 2020, 11, 3943. [Google Scholar] [CrossRef]
- Li, Y.; He, C.; Zuo, L.; Zhao, F.; Zhan, L.; Li, X.; Xia, R.; Yip, H.L.; Li, C.Z.; Liu, X.; et al. High-Performance Semi-Transparent Organic Photovoltaic Devices via Improving Absorbing Selectivity. Adv. Energy Mater. 2021, 11, 2003408. [Google Scholar] [CrossRef]
- Xu, C.; Jin, K.; Xiao, Z.; Zhao, Z.; Ma, X.; Wang, X.; Li, J.; Xu, W.; Zhang, S.; Ding, L.; et al. Wide Bandgap Polymer with Narrow Photon Harvesting in Visible Light Range Enables Efficient Semitransparent Organic Photovoltaics. Adv. Funct. Mater. 2021, 31, 2107934. [Google Scholar] [CrossRef]
- Lee, J.; Jeong, D.; Kim, D.J.; Phan, T.N.; Park, J.S.; Kim, T.; Kim, B.J. Flexible-spacer incorporated polymer donors enable superior blend miscibility for high-performance and mechanically-robust polymer solar cells. Energy Environ. Sci. 2021, 14, 4067–4076. [Google Scholar] [CrossRef]
- Bernardo, G.; Lopes, T.; Lidzey, D.G.; Mendes, A. Progress in Upscaling Organic Photovoltaic Devices. Adv. Energy Mater. 2021, 11, 2100342. [Google Scholar] [CrossRef]
- Brabec, C.J.; Distler, A.; Du, X.; Egelhaaf, H.J.; Hauch, J.; Heumueller, T.; Li, N. Material Strategies to Accelerate OPV Technology Toward a GW Technology. Adv. Energy Mater. 2020, 10, 2001864. [Google Scholar] [CrossRef]
- Xu, Y.; Yao, H.; Ma, L.; Wu, Z.; Cui, Y.; Hong, L.; Zu, Y.; Wang, J.; Woo, H.Y.; Hou, J. Organic photovoltaic cells with high efficiencies for both indoor and outdoor applications. Mater. Chem. Front. 2021, 5, 893–900. [Google Scholar] [CrossRef]
- Yin, Z.; Wei, J.; Zheng, Q. Interfacial Materials for Organic Solar Cells: Recent Advances and Perspectives. Adv. Sci. 2016, 3, 1500362. [Google Scholar] [CrossRef] [Green Version]
- Mishra, A. Interfacial Materials for Organic Solar Cells. In Solar Energy: Systems, Challenges, and Opportunities; Tyagi, H., Chakraborty, P.R., Powar, S., Agarwal, A.K., Eds.; Springer: Singapore, 2020; pp. 373–423. [Google Scholar]
- Kumaresan, P.; Vegiraju, S.; Ezhumalai, Y.; Yau, S.; Kim, C.; Lee, W.; Chen, M. Fused-Thiophene Based Materials for Organic Photovoltaics and Dye-Sensitized Solar Cells. Polymers 2014, 6, 2645–2669. [Google Scholar] [CrossRef]
- Heeger, A.J. 25th Anniversary Article: Bulk Heterojunction Solar Cells: Understanding the Mechanism of Operation. Adv. Mater. 2014, 26, 10–28. [Google Scholar] [CrossRef]
- Gupta, D.; Mukhopadhyay, S.; Narayan, K.S. Fill factor in organic solar cells. Sol. Energy Mater. Sol. Cells 2010, 94, 1309–1313. [Google Scholar] [CrossRef]
- Nagasawa, S.; Al-Naamani, E.; Saeki, A. Computer-Aided Screening of Conjugated Polymers for Organic Solar Cell: Classification by Random Forest. J. Phys. Chem. Lett. 2018, 9, 2639–2646. [Google Scholar] [CrossRef]
- Li, N.; McCulloch, I.; Brabec, C.J. Analyzing the efficiency, stability and cost potential for fullerene-free organic photovoltaics in one figure of merit. Energy Environ. Sci. 2018, 11, 1355–1361. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.; Kirchartz, T.; Vezie, M.S.; Faist, M.A.; Gong, W.; He, Z.; Wu, H.; Troughton, J.; Watson, T.; Bryant, D.; et al. Quantifying Losses in Open-Circuit Voltage in Solution-Processable Solar Cells. Phys. Rev. Appl. 2015, 4, 14020. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Yao, H.; Xu, J.; Hou, J. Recent Progress in Reducing Voltage Loss in Organic Photovoltaic Cells. Mater. Chem. Front. 2021, 5, 709–722. [Google Scholar] [CrossRef]
- Hong, L.; Yao, H.; Wu, Z.; Cui, Y.; Zhang, T.; Xu, Y.; Yu, R.; Liao, Q.; Gao, B.; Xian, K.; et al. Eco-Compatible Solvent-Processed Organic Photovoltaic Cells with Over 16% Efficiency. Adv. Mater. 2019, 31, 1903441. [Google Scholar] [CrossRef]
- Fan, B.; Zhang, D.; Li, M.; Zhong, W.; Zeng, Z.; Ying, L.; Huang, F.; Cao, Y. Achieving over 16% efficiency for single-junction organic solar cells. Sci. China Chem. 2019, 62, 746–752. [Google Scholar] [CrossRef]
- Li, W.; Ye, L.; Li, S.; Yao, H.; Ade, H.; Hou, J. A High-Efficiency Organic Solar Cell Enabled by the Strong Intramolecular Electron Push–Pull Effect of the Nonfullerene Acceptor. Adv. Mater. 2018, 30, 1707170. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, Y.; Zhou, L.; Zhang, C.; Lau, T.K.; Zhang, G.; Lu, X.; Yip, H.L.; So, S.K.; Beaupré, S.; et al. Fused Benzothiadiazole: A Building Block for n-Type Organic Acceptor to Achieve High-Performance Organic Solar Cells. Adv. Mater. 2019, 31, 1807577. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Huang, T.; Cheng, P.; Zou, Y.; Zhang, H.; Yang, J.L.; Chang, S.; Zhang, Z.; Huang, W.; Wang, R.; et al. Enabling low voltage losses and high photocurrent in fullerene-free organic photovoltaics. Nat. Commun. 2019, 10, 570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, P.; Yang, Y. Narrowing the Band Gap: The Key to High-Performance Organic Photovoltaics. Acc. Chem. Res. 2020, 53, 1218–1228. [Google Scholar] [CrossRef]
- Feng, L.; Yuan, J.; Zhang, Z.; Peng, H.; Zhang, Z.; Xu, S.; Liu, Y.; Li, Y.; Zou, Y. Thieno[3,2-b]pyrrolo-Fused Pentacyclic Benzotriazole-Based Acceptor for Efficient Organic Photovoltaics. ACS Appl. Mater. Interfaces 2017, 9, 31985–31992. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Chen, C.; Ho, Y.; Chang, S.; Witek, H.A.; Hsu, C. Thieno[3,2-b]pyrrolo Donor Fused with Benzothiadiazolo, Benzoselenadiazolo and Quinoxalino Acceptors: Synthesis, Characterization, and Molecular Properties. Org. Lett. 2011, 13, 5484–5487. [Google Scholar] [CrossRef]
- Zhang, D.; Fan, B.; Ying, L.; Li, N.; Brabec, C.J.; Huang, F.; Cao, Y. Recent progress in thick-film organic photovoltaic devices: Materials, devices, and processing. SusMat 2021, 1, 4–23. [Google Scholar] [CrossRef]
- Chen, Z.; Cai, P.; Chen, J.; Liu, X.; Zhang, L.; Lan, L.; Peng, J.; Ma, Y.; Cao, Y. Low Band-Gap Conjugated Polymers with Strong Interchain Aggregation and Very High Hole Mobility Towards Highly Efficient Thick-Film Polymer Solar Cells. Adv. Mater. 2014, 26, 2586–2591. [Google Scholar] [CrossRef]
- Lei, T.; Xia, X.; Wang, J.; Liu, C.; Pei, J. “Conformation Locked” Strong Electron-Deficient Poly(p-Phenylene Vinylene) Derivatives for Ambient-Stable n-Type Field-Effect Transistors: Synthesis, Properties, and Effects of Fluorine Substitution Position. J. Am. Chem. Soc. 2014, 136, 2135–2141. [Google Scholar] [CrossRef]
- Huang, H.; Yang, L.; Facchetti, A.; Marks, T.J. Organic and Polymeric Semiconductors Enhanced by Noncovalent Conformational Locks. Chem. Rev. 2017, 117, 10291–10318. [Google Scholar] [CrossRef]
- Zheng, Z.; Yao, H.; Ye, L.; Xu, Y.; Zhang, S.; Hou, J. PBDB-T and its derivatives: A family of polymer donors enables over 17% efficiency in organic photovoltaics. Mater. Today 2020, 35, 115–130. [Google Scholar] [CrossRef]
- Wang, R.; Yuan, J.; Wang, R.; Han, G.; Huang, T.; Huang, W.; Xue, J.; Wang, H.C.; Zhang, C.; Zhu, C.; et al. Rational Tuning of Molecular Interaction and Energy Level Alignment Enables High-Performance Organic Photovoltaics. Adv. Mater. 2019, 31, 1904215. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhan, X. Fused-Ring Electron Acceptors for Photovoltaics and Beyond. Acc. Chem. Res. 2021, 54, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Tang, C.; Ma, Y.; Zhu, X.; Wang, J.; Gao, J.; Xu, C.; Wang, Y.; Zhang, J.; Zheng, Q.; et al. Over 17% Efficiency of Ternary Organic Photovoltaics Employing Two Acceptors with an Acceptor–Donor–Acceptor Configuration. ACS Appl. Mater. Interfaces 2021, 13, 57684–57692. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhong, C.; Su, S.; Xu, M.; Wu, H.; Cao, Y. Enhanced power-conversion efficiency in polymer solar cells using an inverted device structure. Nat. Photonics 2012, 6, 591–595. [Google Scholar] [CrossRef]
- Wang, Y.; Bai, H.; Zhan, X. Comparison of conventional and inverted structures in fullerene-free organic solar cells. J. Energy Chem. 2015, 24, 744–749. [Google Scholar] [CrossRef]
- Fan, B.; Li, M.; Zhang, D.; Zhong, W.; Ying, L.; Zeng, Z.; An, K.; Huang, Z.; Shi, L.; Bazan, G.C.; et al. Tailoring Regioisomeric Structures of π-Conjugated Polymers Containing Monofluorinated π-Bridges for Highly Efficient Polymer Solar Cells. ACS Energy Lett. 2020, 5, 2087–2094. [Google Scholar] [CrossRef]
- Ma, R.; Liu, T.; Luo, Z.; Guo, Q.; Xiao, Y.; Chen, Y.; Li, X.; Luo, S.; Lu, X.; Zhang, M.; et al. Improving open-circuit voltage by a chlorinated polymer donor endows binary organic solar cells efficiencies over 17%. Sci. China Chem. 2020, 63, 325–330. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Jiang, Y.; Jin, K.; Qin, J.; Xu, J.; Li, W.; Xiong, J.; Liu, J.; Xiao, Z.; Sun, K.; et al. 18% Efficiency organic solar cells. Sci. Bull. 2020, 65, 272–275. [Google Scholar] [CrossRef] [Green Version]
- Yan, T.; Song, W.; Huang, J.; Peng, R.; Huang, L.; Ge, Z. 16.67% Rigid and 14.06% Flexible Organic Solar Cells Enabled by Ternary Heterojunction Strategy. Adv. Mater. 2019, 31, 1902210. [Google Scholar] [CrossRef]
- Yu, R.; Yao, H.; Cui, Y.; Hong, L.; He, C.; Hou, J. Improved Charge Transport and Reduced Nonradiative Energy Loss Enable Over 16% Efficiency in Ternary Polymer Solar Cells. Adv. Mater. 2019, 31, 1902302. [Google Scholar] [CrossRef]
- Li, K.; Wu, Y.; Tang, Y.; Pan, M.A.; Ma, W.; Fu, H.; Zhan, C.; Yao, J. Ternary Blended Fullerene-Free Polymer Solar Cells with 16.5% Efficiency Enabled with a Higher-LUMO-Level Acceptor to Improve Film Morphology. Adv. Energy Mater. 2019, 9, 1901728. [Google Scholar] [CrossRef]
- Wang, X.; Sun, Q.; Gao, J.; Wang, J.; Xu, C.; Ma, X.; Zhang, F. Recent Progress of Organic Photovoltaics with Efficiency over 17%. Energies 2021, 14, 4200. [Google Scholar] [CrossRef]
- Xu, W.; Ma, X.; Son, J.H.; Jeong, S.Y.; Niu, L.; Xu, C.; Zhang, S.; Zhou, Z.; Gao, J.; Woo, H.Y.; et al. Smart Ternary Strategy in Promoting the Performance of Polymer Solar Cells Based on Bulk-Heterojunction or Layer-By-Layer Structure. Small 2021, 2104215. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Geng, Y.; Li, J.; Zhao, B.; Guo, Q.; Zhou, E. A-DA′D-A-Type Non-fullerene Acceptors Containing a Fused Heptacyclic Ring for Poly(3-hexylthiophene)-Based Polymer Solar Cells. J. Phys. Chem. C 2020, 124, 24616–24623. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, S.; Ren, J.; Gao, M.; Bi, P.; Ye, L.; Hou, J. Molecular design of a non-fullerene acceptor enables a P3HT-based organic solar cell with 9.46% efficiency. Energy Environ. Sci. 2020, 13, 2864–2869. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Cai, G.; Zhang, Y.; Lu, X.; Lin, Y. Selenium Heterocyclic Electron Acceptor with Small Urbach Energy for As-Cast High-Performance Organic Solar Cells. J. Am. Chem. Soc. 2020, 142, 18741–18745. [Google Scholar] [CrossRef]
- Zhu, C.; An, K.; Zhong, W.; Li, Z.; Qian, Y.; Su, X.; Ying, L. Design and synthesis of non-fullerene acceptors based on a quinoxalineimide moiety as the central building block for organic solar cells. Chem. Commun. 2020, 56, 4700–4703. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Adil, M.A.; Zhang, J.; Wei, Z. Large-Area Organic Solar Cells: Material Requirements, Modular Designs, and Printing Methods. Adv. Mater. 2018, 31, 1805089. [Google Scholar] [CrossRef]
- Zhang, H.; Yao, H.; Hou, J.; Zhu, J.; Zhang, J.; Li, W.; Yu, R.; Gao, B.; Zhang, S.; Hou, J. Over 14% Efficiency in Organic Solar Cells Enabled by Chlorinated Nonfullerene Small-Molecule Acceptors. Adv. Mater. 2018, 30, 1800613. [Google Scholar] [CrossRef]
- Zhang, Y.; Yao, H.; Zhang, S.; Qin, Y.; Zhang, J.; Yang, L.; Li, W.; Wei, Z.; Gao, F.; Hou, J. Fluorination vs. chlorination: A case study on high performance organic photovoltaic materials. Sci. China Chem. 2018, 61, 1328–1337. [Google Scholar] [CrossRef]
- Cai, F.; Zhu, C.; Yuan, J.; Li, Z.; Meng, L.; Liu, W.; Peng, H.; Jiang, L.; Li, Y.; Zou, Y. Efficient organic solar cells based on a new “Y-series” non-fullerene acceptor with an asymmetric electron-deficient-core. Chem. Commun. 2020, 56, 4340–4343. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Fu, H.; Li, Y.; Lin, F.; Sun, R.; Wu, Z.; Wu, X.; Zhong, C.; Min, J.; Luo, J.; et al. Asymmetric Acceptors Enabling Organic Solar Cells to Achieve an over 17% Efficiency: Conformation Effects on Regulating Molecular Properties and Suppressing Nonradiative Energy Loss. Adv. Energy Mater. 2021, 11, 2003177. [Google Scholar] [CrossRef]
- Gao, W.; Fan, B.; Qi, F.; Lin, F.; Sun, R.; Xia, X.; Gao, J.; Zhong, C.; Lu, X.; Min, J.; et al. Asymmetric Isomer Effects in Benzo[c][1,2,5]thiadiazole-Fused Nonacyclic Acceptors: Dielectric Constant and Molecular Crystallinity Control for Significant Photovoltaic Performance Enhancement. Adv. Funct. Mater. 2021, 31, 2104369. [Google Scholar] [CrossRef]
- Chai, G.; Chang, Y.; Zhang, J.; Xu, X.; Yu, L.; Zou, X.; Li, X.; Chen, Y.; Luo, S.; Liu, B.; et al. Fine-tuning of side-chain orientations on nonfullerene acceptors enables organic solar cells with 17.7% efficiency. Energy Environ. Sci. 2021, 14, 3469–3479. [Google Scholar] [CrossRef]
- Ridley, J.; Zerner, M. An intermediate neglect of differential overlap technique for spectroscopy: Pyrrole and the azines. Theor. Chim. Acta 1973, 32, 111–134. [Google Scholar] [CrossRef]
- He, C.; Li, Y.; Liu, Y.; Li, Y.; Zhou, G.; Li, S.; Zhu, H.; Lu, X.; Zhang, F.; Li, C.; et al. Near infrared electron acceptors with a photoresponse beyond 1000 nm for highly efficient organic solar cells. J. Mater. Chem. A 2020, 8, 18154–18161. [Google Scholar] [CrossRef]
- Hai, J.; Zhao, W.; Luo, S.; Yu, H.; Chen, H.; Lu, Z.; Li, L.; Zou, Y.; Yan, H. Vinylene π-bridge: A simple building block for ultra-narrow bandgap nonfullerene acceptors enable 14.2% efficiency in binary organic solar cells. Dye. Pigment. 2021, 188, 109171. [Google Scholar] [CrossRef]
- Jiang, K.; Wei, Q.; Lai, J.Y.L.; Peng, Z.; Kim, H.K.; Yuan, J.; Ye, L.; Ade, H.; Zou, Y.; Yan, H. Alkyl Chain Tuning of Small Molecule Acceptors for Efficient Organic Solar Cells. Joule 2019, 3, 3020–3033. [Google Scholar] [CrossRef]
- Cui, Y.; Yao, H.; Hong, L.; Zhang, T.; Tang, Y.; Lin, B.; Xian, K.; Gao, B.; An, C.; Bi, P.; et al. Organic photovoltaic cell with 17% efficiency and superior processability. Natl. Sci. Rev. 2020, 7, 1239–1246. [Google Scholar] [CrossRef]
- Yu, G.; Gao, J.; Hummelen, J.C.; Wudl, F.; Heeger, A.J. Polymer Photovoltaic Cells: Enhanced Efficiencies via a Network of Internal Donor-Acceptor Heterojunctions. Science 1995, 270, 1789–1791. [Google Scholar] [CrossRef] [Green Version]
- Halls, J.; Walsh, C.A.; Greenham, N.C.; Marseglia, E.A.; Friend, R.H.; Moratti, S.C.; Holmes, A.B. Efficient photodiodes from interpenetrating polymer networks. Nature 1995, 376, 498–500. [Google Scholar] [CrossRef]
- Moore, J.R.; Albert-Seifried, S.; Rao, A.; Massip, S.; Watts, B.; Morgan, D.J.; Friend, R.H.; McNeill, C.R.; Sirringhaus, H. Polymer Blend Solar Cells Based on a High-Mobility Naphthalenediimide-Based Polymer Acceptor: Device Physics, Photophysics and Morphology. Adv. Energy Mater. 2011, 1, 230–240. [Google Scholar] [CrossRef]
- Fan, B.; Ying, L.; Zhu, P.; Pan, F.; Liu, F.; Chen, J.; Huang, F.; Cao, Y. All-Polymer Solar Cells Based on a Conjugated Polymer Containing Siloxane-Functionalized Side Chains with Efficiency over 10%. Adv. Mater. 2017, 29, 1703906. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Zhang, J.; Zhong, W.; Liang, Y.; Zhang, K.; Dong, S.; Ying, L.; Liu, F.; Wang, X.; Huang, F.; et al. 14.4% efficiency all-polymer solar cell with broad absorption and low energy loss enabled by a novel polymer acceptor. Nano Energy 2020, 72, 104718. [Google Scholar] [CrossRef]
- Wang, W.; Wu, Q.; Sun, R.; Guo, J.; Wu, Y.; Shi, M.; Yang, W.; Li, H.; Min, J. Controlling Molecular Mass of Low-Band-Gap Polymer Acceptors for High-Performance All-Polymer Solar Cells. Joule 2020, 4, 1070–1086. [Google Scholar] [CrossRef]
- Yu, H.; Qi, Z.; Yu, J.; Xiao, Y.; Sun, R.; Luo, Z.; Cheung, A.M.H.; Zhang, J.; Sun, H.; Zhou, W.; et al. Fluorinated End Group Enables High-Performance All-Polymer Solar Cells with Near-Infrared Absorption and Enhanced Device Efficiency over 14%. Adv. Energy Mater. 2021, 11, 2003171. [Google Scholar] [CrossRef]
- Peng, F.; An, K.; Zhong, W.; Li, Z.; Ying, L.; Li, N.; Huang, Z.; Zhu, C.; Fan, B.; Huang, F.; et al. A Universal Fluorinated Polymer Acceptor Enables All-Polymer Solar Cells with >15% Efficiency. ACS Energy Lett. 2020, 5, 3702–3707. [Google Scholar] [CrossRef]
- Yu, H.; Pan, M.; Sun, R.; Agunawela, I.; Zhang, J.; Li, Y.; Qi, Z.; Han, H.; Zou, X.; Zhou, W.; et al. Regio-Regular Polymer Acceptors Enabled by Determined Fluorination on End Groups for All-Polymer Solar Cells with 15.2% Efficiency. Angew. Chem. 2021, 133, 10225–10234. [Google Scholar] [CrossRef]
- Yu, H.; Luo, S.; Sun, R.; Angunawela, I.; Qi, Z.; Peng, Z.; Zhou, W.; Han, H.; Wei, R.; Pan, M.; et al. A Difluoro-Monobromo End Group Enables High-Performance Polymer Acceptor and Efficient All-Polymer Solar Cells Processable with Green Solvent under Ambient Condition. Adv. Funct. Mater. 2021, 31, 2100791. [Google Scholar] [CrossRef]
- Sun, R.; Wang, W.; Yu, H.; Chen, Z.; Xia, X.; Shen, H.; Guo, J.; Shi, M.; Zheng, Y.; Wu, Y.; et al. Achieving over 17% efficiency of ternary all-polymer solar cells with two well-compatible polymer acceptors. Joule 2021, 5, 1548–1565. [Google Scholar] [CrossRef]
- Butler, K.T.; Davies, D.W.; Cartwright, H.; Isayev, O.; Walsh, A. Machine learning for molecular and materials science. Nature 2018, 559, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Feng, T. Rectification, space-charge-limited current, photovoltaic and photoconductive properties of Al/tetracene/Au sandwich cell. J. Appl. Phys. 1973, 44, 2781–2788. [Google Scholar] [CrossRef]
- Sun, W.; Zheng, Y.; Yang, K.; Zhang, Q.; Shah, A.A.; Wu, Z.; Sun, Y.; Feng, L.; Chen, D.; Xiao, Z.; et al. Machine learning-assisted molecular design and efficiency prediction for high-performance organic photovoltaic materials. Sci. Adv. 2019, 5, 4275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hachmann, J.; Olivares-Amaya, R.; Atahan-Evrenk, S.; Amador-Bedolla, C.; Sánchez-Carrera, R.S.; Gold-Parker, A.; Vogt, L.; Brockway, A.M.; Aspuru-Guzik, A. The Harvard Clean Energy Project: Large-Scale Computational Screening and Design of Organic Photovoltaics on the World Community Grid. J. Phys. Chem. Lett. 2011, 2, 2241–2251. [Google Scholar] [CrossRef] [Green Version]
- Pyzer-Knapp, E.O.; Li, K.; Aspuru-Guzik, A. Learning from the Harvard Clean Energy Project: The Use of Neural Networks to Accelerate Materials Discovery. Adv. Funct. Mater. 2015, 25, 6495–6502. [Google Scholar] [CrossRef]
- Lopez, S.A.; Pyzer-Knapp, E.O.; Simm, G.N.; Lutzow, T.; Li, K.; Seress, L.R.; Hachmann, J.; Aspuru-Guzik, A. The Harvard organic photovoltaic dataset. Sci. Data 2016, 3, 160086. [Google Scholar] [CrossRef] [Green Version]
- Sahu, H.; Rao, W.; Troisi, A.; Ma, H. Toward Predicting Efficiency of Organic Solar Cells via Machine Learning and Improved Descriptors. Adv. Energy Mater. 2018, 8, 1801032. [Google Scholar] [CrossRef]
- Jørgensen, P.B.; Mesta, M.; Shil, S.; García Lastra, J.M.; Jacobsen, K.W.; Thygesen, K.S.; Schmidt, M.N. Machine learning-based screening of complex molecules for polymer solar cells. J. Chem. Phys. 2018, 148, 241735. [Google Scholar] [CrossRef]
- Padula, D.; Simpson, J.D.; Troisi, A. Combining electronic and structural features in machine learning models to predict organic solar cells properties. Mater. Horiz. 2019, 6, 343–349. [Google Scholar] [CrossRef]
- Sun, W.; Li, M.; Li, Y.; Wu, Z.; Sun, Y.; Lu, S.; Xiao, Z.; Zhao, B.; Sun, K. The Use of Deep Learning to Fast Evaluate Organic Photovoltaic Materials. Adv. Theory Simul. 2019, 2, 1800116. [Google Scholar] [CrossRef] [Green Version]
- Du, X.; Lüer, L.; Heumueller, T.; Wagner, J.; Berger, C.; Osterrieder, T.; Wortmann, J.; Langner, S.; Vongsaysy, U.; Bertrand, M.; et al. Elucidating the Full Potential of OPV Materials Utilizing a High-Throughput Robot-Based Platform and Machine Learning. Joule 2021, 5, 495–506. [Google Scholar] [CrossRef]
- Cambria, E.; White, B. Jumping NLP Curves: A Review of Natural Language Processing Research [Review Article]. IEEE Comput. Intell. Mag. 2014, 9, 48–57. [Google Scholar] [CrossRef]
- Cereto-Massagué, A.; Ojeda, M.J.; Valls, C.; Mulero, M.; Garcia-Vallvé, S.; Pujadas, G. Molecular fingerprint similarity search in virtual screening. Methods 2015, 71, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Durant, J.L.; Leland, B.A.; Henry, D.R.; Nourse, J.G. Reoptimization of MDL Keys for Use in Drug Discovery. J. Chem. Inf. Comput. Sci. 2002, 42, 1273–1280. [Google Scholar] [CrossRef] [Green Version]
- Tovar, A.; Eckert, H.; Bajorath, J. Comparison of 2D Fingerprint Methods for Multiple-Template Similarity Searching on Compound Activity Classes of Increasing Structural Diversity. ChemMedChem 2007, 2, 208–217. [Google Scholar] [CrossRef]
- Rester, U. From virtuality to reality—Virtual screening in lead discovery and lead optimization: A medicinal chemistry perspective. Curr. Opin. Drug Discov. Dev. 2008, 11, 559. [Google Scholar]
- Liang, L.; Liu, M.; Martin, C.; Elefteriades, J.A.; Sun, W. A machine learning approach to investigate the relationship between shape features and numerically predicted risk of ascending aortic aneurysm. Biomech. Model. Mechanobiol. 2017, 16, 1519–1533. [Google Scholar] [CrossRef]
- Keogh, E.; Mueen, A. Curse of Dimensionality. Ind. Eng. Chem. 2009, 29, 48–53. [Google Scholar]











| OSC | Organic Solar Cell | NFA | Non-fullerene Acceptor |
| Power-conversion Efficiency | DFT | Density Functional Theory | |
| HOMO LUMO | Highest Occupied Molecular Orbital Lowest Unoccupied Molecular Orbital | BHJ TEM | Bulk Heterojunction Transmission Electron Microscope |
| GIWAXS | Grazing-incidence Wide-angle X-ray Scattering | ML | Machine Learning |
| Acceptors | (nm) | Matched Donor | JSC (mA cm−2) | VOC (V) | FF (%) | PCE (%) |
|---|---|---|---|---|---|---|
| Y6 | 931 | PM6 | 25.3 | 0.83 | 74.8 | 15.7 |
| H1 | 1016 | PBDB-T | 16.81 | 0.784 | 53 | 6.98 |
| H2 | 1016 | PBDB-T | 24.40 | 0.781 | 69 | 13.15 |
| H3 | 1016 | PBDB-T | 25.84 | 0.757 | 70 | 13.75 |
| BTP-1V-2F | 984 | PM6 | 24.75 | 0.80 | 72 | 14.24 |
| BTP-2V-2F | 1020 | PCE10 | 26.50 | 0.66 | 64 | 11.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, S.; Yap, B.K.; Zhong, Z.; Ying, L. Review on Y6-Based Semiconductor Materials and Their Future Development via Machine Learning. Crystals 2022, 12, 168. https://doi.org/10.3390/cryst12020168
Zhong S, Yap BK, Zhong Z, Ying L. Review on Y6-Based Semiconductor Materials and Their Future Development via Machine Learning. Crystals. 2022; 12(2):168. https://doi.org/10.3390/cryst12020168
Chicago/Turabian StyleZhong, Sijing, Boon Kar Yap, Zhiming Zhong, and Lei Ying. 2022. "Review on Y6-Based Semiconductor Materials and Their Future Development via Machine Learning" Crystals 12, no. 2: 168. https://doi.org/10.3390/cryst12020168