Microstructures and Mechanical Properties of Al-Ti-Zr-Nb-Ta-Mo-V Refractory High-Entropy Alloys with Coherent B2 Nanoprecipitation
Abstract
:1. Introduction
2. Materials and Methods
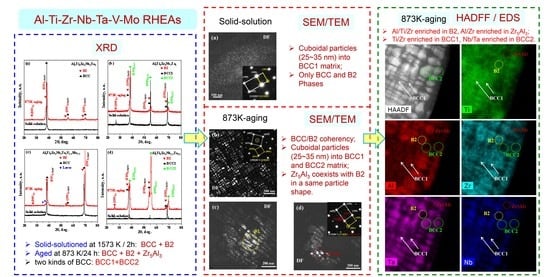
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cantor, B.; Chang, I.; Knight, P.; Vincent, A. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Chen, T.-K.; Shun, T.; Yeh, J.; Wong, M. Nanostructured nitride films of multi-element high-entropy alloys by reactive DC sputtering. Surf. Coat. Technol. 2004, 188–189, 193–200. [Google Scholar] [CrossRef]
- Gao, M.C.; Liaw, P.K.; Yeh, J.W.; Zhang, Y. High-Entropy Alloys; Springer, International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Senkov, O.; Wilks, G.; Scott, J.; Miracle, D. Mechanical properties of Nb25Mo25Ta25W25 and V20Nb20Mo20Ta20W20 refractory high entropy alloys. Intermetallics 2011, 19, 698–706. [Google Scholar] [CrossRef]
- Senkov, O.; Scott, J.M.; Senkova, S.V.; Meisenkothen, F.; Miracle, D.B.; Woodward, C.F. Microstructure and elevated temperature properties of a refractory TaNbHfZrTi alloy. J. Mater. Sci. 2012, 47, 4062–4074. [Google Scholar] [CrossRef]
- Senkov, O.; Senkova, S.; Miracle, D.; Woodward, C. Mechanical properties of low-density, refractory multi-principal element alloys of the Cr–Nb–Ti–V–Zr system. Mater. Sci. Eng. A 2013, 565, 51–62. [Google Scholar] [CrossRef]
- Senkov, O.N.; Isheim, D.; Seidman, D.N.; Pilchak, A.L. Development of a Refractory High Entropy Superalloy. Entropy 2016, 18, 102. [Google Scholar] [CrossRef]
- Senkov, O.N.; Woodward, C.; Miracle, D.B. Microstructure and Properties of Aluminum-Containing Refractory High-Entropy Alloys. JOM 2014, 66, 2030–2042. [Google Scholar] [CrossRef]
- Wu, Y.; Cai, Y.; Chen, X.; Wang, T.; Si, J.; Wang, L.; Wang, Y.; Hui, X. Phase composition and solid solution strengthening effect in TiZrNbMoV high-entropy alloys. Mater. Des. 2015, 83, 651–660. [Google Scholar] [CrossRef]
- Stepanov, N.D.; Yurchenko, N.Y.; Shaysultanov, D.; Salishchev, G.; Tikhonovsky, M.A. Effect of Al on structure and mechanical properties of AlxNbTiVZr (x = 0, 0.5, 1, 1.5) high entropy alloys. Mater. Sci. Technol. 2015, 31, 1184–1193. [Google Scholar] [CrossRef]
- Guo, N.; Wang, L.; Luo, L.; Li, X.; Chen, R.; Su, Y.; Guo, J.; Fu, H. Microstructure and mechanical properties of refractory high entropy (Mo0.5NbHf0.5ZrTi)BCC/M5Si3 in-situ compound. J. Alloys Compd. 2016, 660, 197–203. [Google Scholar] [CrossRef]
- Senkov, O.; Woodward, C. Microstructure and properties of a refractory NbCrMo0.5Ta0.5TiZr alloy. Mater. Sci. Eng. A 2011, 529, 311–320. [Google Scholar] [CrossRef]
- Lei, Z.; Liu, X.; Wu, Y.; Wang, H.; Jiang, S.; Wang, S.; Hui, X.; Wu, Y.; Gault, B.; Kontis, P.; et al. Enhanced strength and ductility in a high-entropy alloy via ordered oxygen complexes. Nat. Cell Biol. 2018, 563, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Senkov, O.; Jensen, J.; Pilchak, A.; Miracle, D.; Fraser, H. Compositional variation effects on the microstructure and properties of a refractory high-entropy superalloy AlMo0.5NbTa0.5TiZr. Mater. Des. 2018, 139, 498–511. [Google Scholar] [CrossRef]
- Soni, V.; Gwalani, B.; Alam, T.; Dasari, S.; Zheng, Y.; Senkov, O.; Miracle, D.; Banerjee, R. Phase inversion in a two-phase, BCC+B2, refractory high entropy alloy. Acta Mater. 2020, 185, 89–97. [Google Scholar] [CrossRef]
- Jensen, J.; Welk, B.; Williams, R.; Sosa, J.; Huber, D.; Senkov, O.; Viswanathan, G.; Fraser, H. Characterization of the microstructure of the compositionally complex alloy Al1Mo0.5Nb1Ta0.5Ti1Zr1. Scr. Mater. 2016, 121, 1–4. [Google Scholar] [CrossRef]
- Soni, V.; Gwalani, B.; Senkov, O.; Viswanathan, B.; Alam, T.; Miracle, D.B.; Banerjee, R. Phase stability as a function of temperature in a refractory high-entropy alloy. J. Mater. Res. 2018, 33, 3235–3246. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, Q.; Jiang, B.; Li, C.; Hao, J.; Li, X.; Dong, C.; Nieh, T. Controlled formation of coherent cuboidal nanoprecipitates in body-centered cubic high-entropy alloys based on Al2(Ni,Co,Fe,Cr)14 compositions. Acta Mater. 2018, 147, 213–225. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, Y.; Jiang, B.; Li, X.; Shi, Y.; Dong, C.; Liaw, P.K. A cuboidal B2 nanoprecipitation-enhanced body-centered-cubic alloy Al0.7CoCrFe2Ni with prominent tensile properties. Scr. Mater. 2016, 120, 85–89. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Wang, Q.; Li, C.; Santodonato, L.J.; Feygenson, M.; Dong, C.; Liaw, P.K. Chemical short-range orders and the induced structural transition in high-entropy alloys. Scr. Mater. 2018, 144, 64–68. [Google Scholar] [CrossRef]
- Li, C.; Ma, Y.; Hao, J.; Yan, Y.; Wang, Q.; Dong, C.; Liaw, P.K. Microstructures and mechanical properties of body-centered-cubic (Al,Ti)0.7(Ni,Co,Fe,Cr)5 high entropy alloys with coherent B2/L21 nanoprecipitation. Mater. Sci. Eng. A 2018, 737, 286–296. [Google Scholar] [CrossRef]
- Ma, Y.; Hao, J.; Wang, Q.; Zhang, C.; Li, C.; Dong, C. Temperature-affected microstructural stability of coherent cuboidal B2 particles in precipitation-strengthened body-centered-cubic Al0.7CoCr2FeNi high-entropy alloy. J. Mater. Sci. 2019, 54, 8696–8710. [Google Scholar] [CrossRef]
- Ma, Y.; Jiang, B.; Li, C.; Wang, Q.; Dong, C.; Liaw, P.K.; Xu, F.; Sun, L. The BCC/B2 Morphologies in AlxNiCoFeCr High-Entropy Alloys. Metals 2017, 7, 57. [Google Scholar] [CrossRef] [Green Version]
- Cullity, D.B.; Stock, S.R. Elements of X-ray Diffraction, 3rd ed.; Prentice Hall: Hoboken, NJ, USA, 2001. [Google Scholar]
- Sosa, J.M.; Jensen, J.K.; Huber, D.E.; Viswanathan, G.B.; Gibson, M.A.; Fraser, H.L. Three-dimensional characterisation of the microstructure of an high entropy alloy using STEM/HAADF tomography. Mater. Sci. Technol. 2015, 31, 1250–1258. [Google Scholar] [CrossRef]
- Wang, Q.; Han, J.C.; Zhang, Z.W.; Dong, C.; Liaw, P.K. Coherent precipitation and stability of cuboidal nanoparticles in body-centered-cubic Al0.4Nb0.5Ta0.5TiZr0.8 refractory high entropy alloy. Scr. Mater. 2021, 190, 40–45. [Google Scholar] [CrossRef]
- Bendersky, L.; Boettinger, W.; Burton, B.; Biancaniello, F.; Shoemaker, C. The formation of ordered ω-related phases in alloys of composition Ti4Al3Nb. Acta Met. Mater. 1990, 38, 931–943. [Google Scholar] [CrossRef]
- Cramer, S.D.; Covino, B.S., Jr. ASM Handbook; ASM International: Almere, The Netherlands, 2005; Volume 13B. [Google Scholar]
- Voorhees, P.; McFadden, G.; Johnson, W. On the morphological development of second-phase particles in elastically-stressed solids. Acta Met. Mater. 1992, 40, 2979–2992. [Google Scholar] [CrossRef]
- Thompson, M.; Su, C.; Voorhees, P. The equilibrium shape of a misfitting precipitate. Acta Met. Mater. 1994, 42, 2107–2122. [Google Scholar] [CrossRef] [Green Version]
- Soni, V.; Senkov, O.N.; Gwalani, B.; Miracle, D.B.; Banerjee, R. Microstructural Design for Improving Ductility of An Initially Brittle Refractory High Entropy Alloy. Sci. Rep. 2018, 8, 8816. [Google Scholar] [CrossRef] [Green Version]
- Soni, V.; Senkov, O.; Couzinie, J.-P.; Zheng, Y.; Gwalani, B.; Banerjee, R. Phase stability and microstructure evolution in a ductile refractory high entropy alloy Al10Nb15Ta5Ti30Zr40. Materialia 2020, 9, 100569. [Google Scholar] [CrossRef]






| No. | Nominal Formula (Composition in at. %) | Phase Constitutions | a (nm) | ε (%) | r (nm) | σYS (MPa) |
|---|---|---|---|---|---|---|
| S0 | Al2Ti4Zr4Nb3Ta3 Al12.5Ti25.0Zr25.0Nb18.75Ta18.75 | BCC + B2 + Zr5Al3 | aBCC = 0.3298 aB2 = 0.3390 | εBCC1/B2 = 2.75 | 20~35 | 1400 |
| S1 | Al2Ti4Zr4Nb5Ta1 Al12.5Ti25.0Zr25.0Nb31.25Ta6.25 | BCC1 + BCC2 + B2+ Zr5Al3 | aBCC1 = 0.3391 aBCC1 = 0.3439 aB2 = 0.3462 | εBCC1/B2 = 2.07 εBCC2/B2 = 0.67 | 25~55 | 1125 |
| S2 | Al2Ti4Zr4Nb3Ta2V0.5Mo0.5Al12.5 Ti25.0Zr25.0Nb18.75Ta12.5 V3.125Mo3.125 | BCC + B2 + Zr5Al3 | aBCC = 0.3294 aB2 = 0.3327 | εBCC1/B2 = 1.00 | 25~45 | 1150 |
| S3 | Al2Ti5Zr4Nb2.5Ta2.5 Al12.5Ti31.25Zr25.0Nb15.625Ta15.625 | BCC1 + BCC 2 + B2 + Zr5Al3 | aBCC1 = 0.3352 aBCC2 = 0.3307 aB2 = 0.3377 | εBCC1/B2 = 2.09 εBCC2/B2 = 0.74 | 25~50 | 1232 |
| Heat Treatment | Phase Constitution | Elemental Composition in Phase (at. %) | ||||
|---|---|---|---|---|---|---|
| Al | Nb | Ta | Ti | Zr | ||
| 873 K/24 h | BCC1 | 5.4 ± 1.3 | 9.1 ± 2.6 | 2.2 ± 1.0 | 53.0 ± 4.5 | 30.3 ± 0.4 |
| BCC2 | 7.9 ± 2.7 | 26.0 ± 2.5 | 22.6 ± 1.7 | 33.4 ± 2.0 | 10.1 ± 1.2 | |
| B2 | 30.0 ± 1.2 | 12.7 ± 0.7 | 2.4 ± 0.7 | 21.4 ± 3.5 | 33.5 ± 3.0 | |
| Zr5Al3 | 39.4 ± 2.3 | 11.1 ± 1.5 | 1.5 ± 0.5 | 9.1 ± 3.1 | 38.9 ± 0.7 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Jin, D.; Han, J.; Wang, Q.; Zhang, Z.; Dong, C. Microstructures and Mechanical Properties of Al-Ti-Zr-Nb-Ta-Mo-V Refractory High-Entropy Alloys with Coherent B2 Nanoprecipitation. Crystals 2021, 11, 833. https://doi.org/10.3390/cryst11070833
Wang Z, Jin D, Han J, Wang Q, Zhang Z, Dong C. Microstructures and Mechanical Properties of Al-Ti-Zr-Nb-Ta-Mo-V Refractory High-Entropy Alloys with Coherent B2 Nanoprecipitation. Crystals. 2021; 11(7):833. https://doi.org/10.3390/cryst11070833
Chicago/Turabian StyleWang, Zhenhua, Dongming Jin, Jincan Han, Qing Wang, Zhongwei Zhang, and Chuang Dong. 2021. "Microstructures and Mechanical Properties of Al-Ti-Zr-Nb-Ta-Mo-V Refractory High-Entropy Alloys with Coherent B2 Nanoprecipitation" Crystals 11, no. 7: 833. https://doi.org/10.3390/cryst11070833