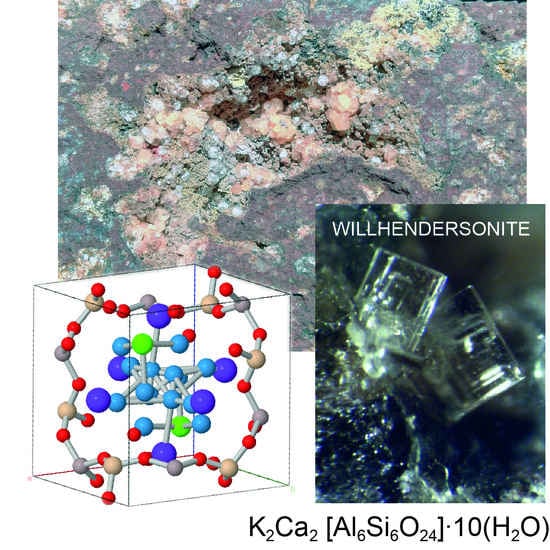
First Occurrence of Willhendersonite in the Lessini Mounts, Northern Italy
Abstract
:1. Introduction
2. Geological Background
3. Materials and Methods
4. Results
5. Discussion and Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Peacor, D.R.; Dunn, P.J.; Simmons, W.B.; Tillmanns, E.; Fischer, R.X. Willhendersonite, a new zeolite isostructural with chabazite. Am. Mineral. 1984, 69, 186–189. [Google Scholar]
- Tillmanns, E.; Fischer, R.X.; Baur, W.H. Chabazite-type framework in the new zeolite willhendersonite, KCaAl3Si3O12·5H2O. Neues Jahrb. Mineral. Mon. 1984, 547–558. [Google Scholar]
- Tillmanns, E.; Fischer, R.X. Über ein neues Zeolithmineral mit geordnetem Chabasitgerüst. Z. Krist. 1982, 159, 125–126. [Google Scholar]
- Gottardi, G.; Galli, E. Natural Zeolites; Springer: Berlin/Heidelberg, Germany, 1985. [Google Scholar]
- Passaglia, E.; Sheppard, R.A. The Crystal Chemistry of Zeolites. In Reviews in Mineralogy and Geochemistry; Bish, D.L., Ming, D.W., Eds.; Mineralogical Society of America: Washington, DC, USA, 2001; Volume 45, pp. 69–116. [Google Scholar]
- Baerlocher, C.; McCusker, L.B. Database of Zeolite Structures, Published by the International Zeolite Association, Hosted at Lab. Crystallography, ETH Zürich, Switzerland. 2007. Available online: http://www.iza-structure.org/databases/ (accessed on 10 February 2017).
- Gottardi, G. Mineralogy and crystal chemistry of zeolites. In Natural Zeolites; Sand, L.B., Mumpton, F.A., Eds.; Pergamon Press: Oxford, UK, 1978; pp. 31–44. [Google Scholar]
- Bish, D.L.; Ming, D.W. Natural Zeolites: Occurrence, Properties, Applications. In Reviews in Mineralogy and Geochemistry; Bish, D.L., Ming, D.W., Eds.; Mineralogical Society of America: Washington, DC, USA, 2001; Volume 45, 654p. [Google Scholar]
- Walter, F.; Postl, W. Willhendersonit vom Stradner Kogel, südlich Gleichenberg, Steiermark. Mitt. Abt. Miner. Landesmus. Joanneum 1984, 52, 39–43. [Google Scholar]
- Vezzalini, G.; Quartieri, S.; Galli, E. Occurrence and crystal structure of a Ca-pure willhendersonite. Zeolites 1997, 19, 75–79. [Google Scholar] [CrossRef]
- Fischer, R.X.; Kahlenberg, V.; Lengauer, C.L.; Tillmanns, E. Thermal behavior and structural transformation in the chabazite-type zeolite willhendersonite, KCaAl3Si3O12·5H2O. Am. Mineral. 2008, 93, 1317–1325. [Google Scholar] [CrossRef]
- Mattioli, M.; Cenni, M.; Passaglia, E. Secondary mineral assemblages as indicators of multi stage alteration processes in basaltic lava flows: Evidence from the Lessini Mountains, Veneto Volcanic Province, Northern Italy. Per. Mineral. 2016, 85, 1–24. [Google Scholar]
- Galli, E. La phillipsite barifera (“wellsite”) di M. Calvarina (Verona). Per. Mineral. 1972, 41, 23–33. [Google Scholar]
- Passaglia, E.; Tagliavini, A.; Gutoni, R. Offretite and other zeolites from Fittà (Verona, Italy). Neues Jahrb. Mineral. Mon. 1996, 418–428. [Google Scholar]
- Giordani, M.; Mattioli, M.; Dogan, M.; Dogan, A.U. Potential carcinogenic erionite from Lessini Mounts, NE Italy: Morphological, mineralogical and chemical characterization. J. Toxicol. Environ. Health A 2016, 79, 808–824. [Google Scholar] [CrossRef]
- Cangiotti, M.; Battistelli, M.; Salucci, S.; Falcieri, E.; Mattioli, M.; Giordani, M.; Ottaviani, M.F. Electron paramagnetic resonance and transmission electron microscopy study of the interactions between asbestiform zeolite fibers and model membranes. J. Toxicol. Environ. Health A 2017, 80, 171–187. [Google Scholar] [CrossRef] [PubMed]
- Giordani, M.; Mattioli, M.; Ballirano, P.; Pacella, P.; Cenni, M.; Boscardin, M.; Valentini, L. Geological occurrence, mineralogical characterization and risk assessment of potentially carcinogenic erionite in Italy. J. Toxicol. Environ. Health B 2017, 20, 81–103. [Google Scholar] [CrossRef] [PubMed]
- Mattioli, M.; Giordani, M.; Arcangeli, P.; Valentini, L.; Boscardin, M.; Pacella, A.; Ballirano, P. Prismatic to Asbestiform Offretite from Northern Italy: Occurrence, Morphology and Crystal-Chemistry of a New Potentially Hazardous Zeolite. Minerals 2018, 8, 69. [Google Scholar] [CrossRef] [Green Version]
- Barbieri, G.; De Zanche, V.; Sedea, R. Vulcanismo Paleogenico ed evoluzione del semigraben Alpone-Agno (Monti Lessini). Rend. Soc. Geol. It. 1991, 14, 5–12. [Google Scholar]
- De Vecchi, G.P.; Sedea, R. The Paleogene basalt of the Veneto region (NE Italy). Mem. Inst. Geol. Min. Univ. Padua 1995, 47, 253–274. [Google Scholar]
- Bonadiman, C.; Coltorti, M.; Milani, L.; Salvini, L.; Siena, F.; Tassinari, R. Metasomatism in the lithospheric mantle and its relationships to magmatism in the Veneto Volcanic Province, Italy. Per. Mineral. 2001, 70, 333–357. [Google Scholar]
- Barbieri, G.; De Zanche, V.; Medizza, F.; Sedea, R. Considerazioni sul vulcanismo terziario del Veneto occidentale e del Trentino meridionale. Rend. Soc. Geol. It. 1982, 4, 267–270. [Google Scholar]
- Zampieri, D. Tertiary extension in the southern Trento Platform, Southern Alps, Italy. Tectonics 1995, 14, 645–657. [Google Scholar] [CrossRef]
- Zampieri, D. Segmentation and linkage of the Lessini Mountains normal Faults, Southern Alps, Italy. Tectonophysics 2000, 319, 19–31. [Google Scholar] [CrossRef]
- Milani, L.; Beccaluva, L.; Coltorti, M. Petrogenesis and evolution of the Euganean magmatic complex, Veneto Region, North-East Italy. Eur. J. Mineral. 1999, 11, 379–399. [Google Scholar] [CrossRef]
- Beccaluva, L.; Bonadiman, C.; Coltorti, M.; Salvini, L.; Siena, F. Depletion events, nature of metasomatizing agent and timing of enrichment processes in lithospheric mantle xenoliths from the Veneto Volcanic Province. J. Petrol. 2001, 42, 173–187. [Google Scholar] [CrossRef]
- Passaglia, E. The crystal chemistry of chabazites. Am. Mineral. 1970, 55, 1278–1301. [Google Scholar]
- Hawkins, D.B. Kinetics of glass dissolution and zeolite formation under hydrothermal conditions. Clays Clay Miner. 1981, 29, 331–340. [Google Scholar] [CrossRef]




| 2 Theta | I/Io | d (Å) | h | k | l | 2 Theta | I/Io | d (Å) | h | k | l |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 9.32 | 34.77 | 9.4789 | 0 | 0 | 1 | 27.36 | 6.15 | 3.2601 | −2 | 2 | 0 |
| 9.61 | 97.78 | 9.1915 | 0 | 1 | 0 | 28.89 | 6.17 | 3.0871 | 0 | 0 | 3 |
| 9.64 | 100 | 9.1591 | 1 | 0 | 0 | 29.47 | 20.59 | 3.0275 | 1 | 2 | 2 |
| 13.63 | 13.46 | 6.4914 | 1 | 1 | 0 | 30.04 | 22.37 | 2.9664 | 1 | 0 | 3 |
| 17.11 | 23.01 | 5.1774 | 1 | 1 | 1 | 30.75 | 56.75 | 2.9054 | −1 | −1 | 3 |
| 18.83 | 9.99 | 4.7407 | 0 | 0 | 2 | 31.42 | 8.74 | 2.8458 | 0 | 3 | 1 |
| 19.41 | 10.24 | 4.5979 | 2 | 0 | 0 | 31.77 | 15.93 | 2.8171 | 3 | 0 | 1 |
| 20.72 | 15.93 | 4.2871 | 0 | −1 | 2 | 31.91 | 35.21 | 2.8028 | −3 | 1 | 1 |
| 21.72 | 27.29 | 4.0873 | −1 | 2 | 0 | 34.86 | 9.71 | 2.5741 | −2 | −2 | 2 |
| 21.68 | 25.71 | 4.1202 | −2 | 1 | 0 | 35.47 | 15.13 | 2.5201 | 1 | −3 | 2 |
| 22.63 | 16.21 | 3.9247 | −1 | −1 | 2 | 36.86 | 7.21 | 2.4384 | 1 | 2 | 3 |
| 23.16 | 23.16 | 3.8546 | −2 | −1 | 1 | 39.46 | 7.05 | 2.2814 | −1 | −1 | 4 |
| 23.46 | 25.29 | 3.8262 | 1 | −1 | 2 | 41.55 | 8.75 | 2.1717 | −3 | 3 | 0 |
| 23.86 | 17.57 | 3.7104 | −1 | 2 | 1 | 44.22 | 6.95 | 2.0464 | −4 | 1 | 2 |
| 23.96 | 15.76 | 3.7461 | 2 | −1 | 1 | 49.47 | 6.97 | 1.8408 | 0 | 1 | 5 |
| 26.36 | 7.09 | 3.3813 | −2 | 0 | 2 | 54.53 | 7.26 | 1.6863 | 0 | −4 | 4 |
| WIL1 | WIL2 | WIL5 | WIL8 | WIL9 | WIL10 | WIL14 | WIL15 | WIL19 | WIL20 | Average | St-d | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca-rich willhendersonite | ||||||||||||
| SiO2 | 35.34 | 35.31 | 34.68 | 35.12 | 35.74 | 34.99 | 35.51 | 35.73 | 34.85 | 34.88 | 35.22 | 0.37 |
| Al2O3 | 29.41 | 29.31 | 29.55 | 29.16 | 28.89 | 29.13 | 29.45 | 28.87 | 29.46 | 29.44 | 29.27 | 0.24 |
| K2O | 2.75 | 2.65 | 2.88 | 2.31 | 2.55 | 3.01 | 2.13 | 2.65 | 2.45 | 2.56 | 2.59 | 0.26 |
| CaO | 13.24 | 13.36 | 13.75 | 13.84 | 13.01 | 12.96 | 13.22 | 13.24 | 13.45 | 13.35 | 13.34 | 0.28 |
| Na2O | 0.15 | 0.27 | 0.13 | 0.37 | 0.25 | 0.25 | 0.12 | 0.18 | 0.12 | 0.15 | 0.20 | 0.08 |
| Fe2O3 | 0.12 | 0.08 | 0.11 | 0.2 | 0.08 | 0.05 | 0.12 | 0.11 | 0.05 | 0.04 | 0.10 | 0.05 |
| H2O * | 18.99 | 19.02 | 18.90 | 19.00 | 19.48 | 19.61 | 19.45 | 19.22 | 19.62 | 19.58 | 19.29 | 0.29 |
| Total | 81.01 | 80.98 | 81.10 | 81.00 | 80.52 | 80.39 | 80.55 | 80.78 | 80.38 | 80.42 | 80.79 | 0.27 |
| Cation on the basis of 12 framework oxygens | Average | St-d | ||||||||||
| Si | 3.05 | 3.05 | 3.05 | 3.04 | 3.13 | 3.05 | 3.07 | 3.09 | 3.03 | 3.04 | 3.06 | 0.03 |
| Al | 3.00 | 2.99 | 3.06 | 2.97 | 2.99 | 2.99 | 3.00 | 2.94 | 3.02 | 3.09 | 3.00 | 0.04 |
| Fe3+ | 0.01 | 0.00 | 0.01 | 0.01 | 0.01 | 0.00 | 0.01 | 0.01 | 0.00 | 0.00 | 0.01 | 0.00 |
| ƩT | 6.06 | 6.04 | 6.11 | 6.03 | 6.12 | 6.05 | 6.08 | 6.04 | 6.06 | 6.13 | 6.07 | 0.04 |
| Ca | 1.23 | 1.24 | 1.29 | 1.28 | 1.22 | 1.21 | 1.22 | 1.23 | 1.25 | 1.24 | 1.24 | 0.03 |
| K | 0.30 | 0.29 | 0.31 | 0.26 | 0.29 | 0.34 | 0.24 | 0.29 | 0.27 | 0.28 | 0.29 | 0.03 |
| Na | 0.03 | 0.05 | 0.02 | 0.06 | 0.04 | 0.04 | 0.02 | 0.03 | 0.02 | 0.03 | 0.03 | 0.01 |
| ƩC | 1.55 | 1.58 | 1.63 | 1.60 | 1.55 | 1.59 | 1.48 | 1.55 | 1.55 | 1.55 | 1.56 | 0.04 |
| Total | 7.61 | 7.62 | 7.74 | 7.63 | 7.68 | 7.64 | 7.56 | 7.59 | 7.60 | 7.68 | 7.63 | 0.06 |
| E% | 0.09 | 0.08 | 0.05 | 0.05 | 0.09 | 0.09 | 0.12 | 0.07 | 0.09 | 0.11 | ||
| Ca/(Ca + K) | 0.80 | 0.81 | 0.81 | 0.83 | 0.81 | 0.78 | 0.84 | 0.81 | 0.82 | 0.81 | 0.81 | 0.02 |
| R | 0.50 | 0.51 | 0.50 | 0.51 | 0.51 | 0.50 | 0.51 | 0.51 | 0.50 | 0.50 | 0.51 | 0.00 |
| WIL4 | WIL7 | WIL12 | WIL13 | WIL22 | WIL23 | WIL24 | WIL27 | WIL29 | WIL31 | Average | St-d | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca-K willhendersonite | ||||||||||||
| SiO2 | 34.81 | 34.45 | 34.86 | 34.91 | 34.23 | 34.57 | 35.02 | 34.96 | 35.12 | 34.82 | 34.78 | 0.28 |
| Al2O3 | 28.12 | 28.42 | 28.09 | 28.02 | 28.66 | 28.38 | 27.86 | 27.98 | 27.87 | 28.46 | 28.19 | 0.28 |
| K2O | 8.11 | 8.45 | 8.66 | 8.12 | 7.99 | 8.13 | 8.55 | 8.71 | 8.05 | 8.44 | 8.32 | 0.27 |
| CaO | 10.75 | 10.42 | 10.25 | 10.77 | 11.02 | 10.79 | 10.35 | 10.16 | 10.22 | 10.46 | 10.52 | 0.29 |
| Na2O | 0.04 | 0.05 | 0.02 | 0.04 | 0.01 | 0.06 | 0.08 | 0.04 | 0.04 | 0.01 | 0.04 | 0.02 |
| Fe2O3 | 0.05 | 0.01 | 0.02 | 0.04 | 0.05 | 0.01 | 0.03 | 0.06 | 0.01 | 0.01 | 0.03 | 0.02 |
| H2O * | 18.12 | 18.20 | 18.10 | 18.10 | 18.04 | 18.06 | 18.11 | 18.09 | 18.69 | 17.80 | 18.13 | 0.22 |
| Total | 81.88 | 81.80 | 81.90 | 81.90 | 81.96 | 81.94 | 81.89 | 81.91 | 81.31 | 82.20 | 81.87 | 0.22 |
| Cation on the basis of 12 framework oxygens | Average | St-d | ||||||||||
| Si | 3.07 | 3.05 | 3.08 | 3.08 | 3.02 | 3.05 | 3.09 | 3.09 | 3.11 | 3.06 | 3.07 | 0.03 |
| Al | 2.92 | 2.96 | 2.92 | 2.91 | 2.98 | 2.95 | 2.90 | 2.91 | 2.91 | 2.95 | 2.93 | 0.03 |
| Fe3+ | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| ƩT | 5.99 | 6.01 | 6.00 | 5.99 | 6.00 | 6.00 | 5.99 | 6.00 | 6.01 | 6.01 | 6.00 | 0.01 |
| Ca | 1.02 | 0.99 | 0.97 | 1.02 | 1.04 | 1.02 | 0.98 | 0.96 | 0.97 | 0.99 | 0.99 | 0.03 |
| K | 0.91 | 0.95 | 0.98 | 0.91 | 0.90 | 0.91 | 0.96 | 0.98 | 0.91 | 0.95 | 0.94 | 0.03 |
| Na | 0.01 | 0.01 | 0.00 | 0.01 | 0.00 | 0.01 | 0.01 | 0.01 | 0.01 | 0.00 | 0.01 | 0.00 |
| ƩC | 1.93 | 1.95 | 1.95 | 1.94 | 1.94 | 1.94 | 1.96 | 1.95 | 1.89 | 1.93 | 1.94 | 0.02 |
| Total | 7.93 | 7.96 | 7.95 | 7.93 | 7.94 | 7.94 | 7.95 | 7.95 | 7.90 | 7.94 | 7.94 | 0.02 |
| E% | −0.01 | 0.01 | 0.00 | −0.01 | 0.00 | 0.00 | −0.01 | 0.00 | 0.02 | 0.01 | ||
| Ca/(Ca + K) | 0.53 | 0.51 | 0.50 | 0.53 | 0.54 | 0.53 | 0.50 | 0.49 | 0.52 | 0.51 | 0.51 | 0.01 |
| R | 0.51 | 0.51 | 0.51 | 0.51 | 0.50 | 0.51 | 0.52 | 0.51 | 0.52 | 0.51 | 0.51 | 0.00 |
| a (Å) | b (Å) | c (Å) | α (°) | β (°) | γ (°) | V (Å3) | |
| Lessini (this work) | 9.239(2) | 9.221(2) | 9.496(2) | 92.324(2) | 92.677(2) | 89.992(2) | 808.9(5) |
| Literature data | |||||||
| Terni [1] | 9.138 | 9.178 | 9.477 | 92.31 | 92.50 | 90.05 | 793.4 |
| Terni [10] | 9.180(3) | 9.197(3) | 9.440(3) | 91.42(2) | 91.72(2) | 90.05(2) | 796.4(5) |
| Styria [9] | 9.16 | 9.17 | 9.49 | 92.3 | 92.9 | 90.4 | 795.4 |
| Mayen [1] | 9.21(2) | 9.23(2) | 9.52(2) | 92.4(1) | 92.7(1) | 90.1(1) | 807.7(30) |
| Mayen [2] | 9.206(2) | 9.216(2) | 9.500(4) | 92.34(3) | 92.70(3) | 90.12(3) | 804.4(4) |
| Bellberg [11] | 9.248(5) | 9.259(5) | 9.533(5) | 92.313(5) | 92.761(5) | 89.981(5) | 814.7(8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mattioli, M.; Cenni, M. First Occurrence of Willhendersonite in the Lessini Mounts, Northern Italy. Crystals 2021, 11, 109. https://doi.org/10.3390/cryst11020109
Mattioli M, Cenni M. First Occurrence of Willhendersonite in the Lessini Mounts, Northern Italy. Crystals. 2021; 11(2):109. https://doi.org/10.3390/cryst11020109
Chicago/Turabian StyleMattioli, Michele, and Marco Cenni. 2021. "First Occurrence of Willhendersonite in the Lessini Mounts, Northern Italy" Crystals 11, no. 2: 109. https://doi.org/10.3390/cryst11020109