Preparation and Evaluation of Cu-Zn-GNSs Nanocomposite Manufactured by Powder Metallurgy
Abstract
:1. Introduction
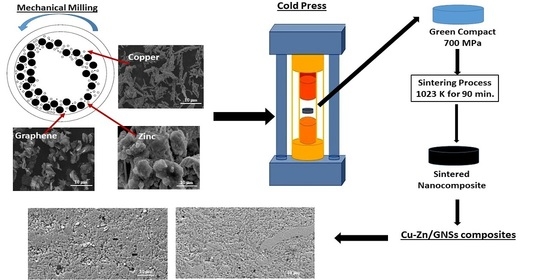
2. Experimental Work
3. Results and Discussions
3.1. Optical Micrographs
3.2. Microstructure Examination
3.3. EDX Analysis
3.4. Relative Density
3.5. XRD Analysis
3.6. Vickers Hardness
3.7. Compression Strengths Estimation
3.8. Electrical Conductivity Measurements
3.9. Thermal Conductivity Estimation
3.10. Wear Behavior
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yusoff, M.; Mohamad, M.; Abu Bakar, M.B.; Masri, M.N.; Noriman, N.Z.; Dahham, O.S.; Umar, M.U. Copper Alloy Reinforced by Graphene by Powder Metallurgy Technique. AIP Conf. Proc. 2020, 2213, 020264. [Google Scholar]
- Pola, A.; Rollez, D.; Prenger, F. Zinc Alloy Family for Foundry Purposes. World Metall. Erzmetall 2015, 68, 354–358. [Google Scholar]
- Yang, G.S.; Lee, J.K.; Jang, W.Y. Mechanical Properties and Microstructure Observation with Grain Refinement in CuZnAl Alloy. Mater. Sci. Forum Jan. 2008, 569, 173–176. [Google Scholar] [CrossRef]
- Rajeshkumara, L.; Suriyanarayanan, R.; Hari, K.S.; Babu, S.V.; Bhuvaneswari, V.; Karunan, M.P.J. Influence of boron carbide addition on particle size of copper zinc alloys synthesized by powders metallurgy. Mater. Sci. Eng. 2020, 954, 1–9. [Google Scholar] [CrossRef]
- Traleski, A.V.; Vurobi, S., Jr.; Cintho, O.M. Osvaldo Mitsuyuki Cintho, Processing of Cu-Al-Ni and Cu-Zn-Al Alloys by Mechanical Alloying. Mat. Sci. Forum 2012, 727–728, 200–205. [Google Scholar]
- Da Silva, F.C.; Kazmierczak, K.; da Costa, C.-E.; Milan, J.C.G.; Torralba, J.M. Zamak 2 Alloy Produced by Mechanical Alloying and Consolidated by Sintering and Hot Pressing. J. Manuf. Sci. Eng. 2017, 139, 1–7. [Google Scholar] [CrossRef]
- Imai, H.; Li, S.; Kondoh, K.; Kosaka, Y.; Okada, K.; Yamamoto, K.; Takahashi, M.; Umeda, J. Microstructure and Mechanical Properties of Cu-40%Zn-0.5%Cr Alloy by Powder Metallurgy. Mater. Trans. 2014, 55, 528–533. [Google Scholar] [CrossRef] [Green Version]
- Global Zinc Market to Grow at 3.8% in 2022. Available online: https://www.mining-technology.com/comment/zinc-outlook-2019/ (accessed on 5 January 2020).
- Lynch, R.F. Zinc: Alloying, Thermomechanical Processing, Properties, and Applications. In Encyclopedia of Materials: Science and Technology; Elsevier: Amsterdam, The Netherlands, 2001; pp. 9869–9883. [Google Scholar]
- Marder, A.R. Metallurgy of zinc-coated steel. Prog. Mater. Sci. 2000, 45, 191–271. [Google Scholar] [CrossRef]
- Levy, G.K.; Goldman, J.; Aghion, E. The prospects of zinc as a structural material for biodegradable implants: A review paper. Metals 2017, 7, 402. [Google Scholar] [CrossRef] [Green Version]
- Pola, A.; Tocci, M.; Goodwin, F.E. Review of Microstructures and Properties of Zinc Alloys. Metals 2020, 10, 253. [Google Scholar] [CrossRef] [Green Version]
- Panigrahi, M.; Avar, B. Influence of mechanical alloying on structural, thermal, and magnetic properties of Fe50Ni10Co10Ti10B20 high entropy soft magnetic alloy. J. Mater. Sci. Mater. Electron. 2021, 32, 21124–21134. [Google Scholar] [CrossRef]
- Wang, K.J.; Cai, X.L.; Wang, H.; Hu, J.; Zhang, Y.F. Preparation of Cu-Zn Alloy by Different High Energy Ball Milling. Adv. Mater. Res. 2012, 12, 259–262. [Google Scholar]
- Kaijun, W. Preparation of Cu-Zn Alloy by High Energy Ball Milling of Elemental Copper and Zinc. Adv. Mater. Res. 2011, 148–149, 1413–1416. [Google Scholar]
- El-Khatib, S.; Shash, A.Y.; Elsayed, A.H.; El-Habak, A. Effect of carbon nano-tubes and dispersions of SiC and Al2O3 on the mechanical and physical properties of copper-nickel alloy. Heliyon 2018, 4, e00876. [Google Scholar] [CrossRef]
- Zhi-Chao, M.A. Effects of zinc on static and dynamic mechanical properties of copper-zinc alloy. J. Cent. South Univ. 2015, 22, 2440–2445. [Google Scholar]
- Hamed, A.; Mosa, E.; Mahdy, A.; El-Batanony, I.; Alkady, O. Effect of process controlling agent on the microstructure, and mechanical properties of copper/graphene composite. In Proceedings of the Al Azhar Engineering Fifteenth International Conference, Cairo, Egypt, 13–15 March 2021. [Google Scholar]
- Fathy, A.; Elkady, O.; Abu-Oqail, A. Microstructure, mechanical and wear properties of Cu-ZrO2 nanocomposites. Mater. Sci. Technol. 2017, 33–17, 2138–2146. [Google Scholar] [CrossRef]
- Yehia, H.M. Effect of graphene nano-sheets content and sintering time on the microstructure, coefficient of thermal expansion, and mechanical properties of (Cu/WC-TiC-Co) nano-composites. J. Alloys Compd. 2018, 764, 36–43. [Google Scholar] [CrossRef]
- Fathy, A.; Elkady, O.; Abu-Oqail, A. Synthesis and characterization of Cu-ZrO2 nanocomposite produced by thermochemical process. J. Alloys Compd. 2017, 719, 411–419. [Google Scholar] [CrossRef]
- Fathy, A.; Elkady, O.; Abu-Oqail, A. Effect of high energy ball milling on strengthening of Cu-ZrO2 nanocomposites. Ceram. Int. 2019, 45, 5866–5875. [Google Scholar] [CrossRef]
- El-Kady, O.; Yehia, H.M.; Nouh, F. Preparation and characterization of Cu/(WC-TiC-Co)/graphene nanocomposites as a suitable material for heat sink by powder metallurgy method. Int. J. Refract. Met. Hard Mater. 2019, 79, 108–114. [Google Scholar] [CrossRef]
- Elkady, O.A.M.; Abu-Oqail, A.; Ewais, E.M.M.; El-Sheikh, M. Physico-mechanical and tribological properties of Cu/h-BN nanocomposites synthesized by PM route. J. Alloys Compd. 2015, 625, 309–317. [Google Scholar] [CrossRef]
- Abu-Oqail, A.; Wagih, A.; Fathy, A.; Elkady, O.; Kabeel, A.M. Production and properties of Cu-ZrO2 nanocomposites. J. Compos. Mater. 2017, 52–11, 1519–1529. [Google Scholar]
- Yehia, H.M.; Abu-Oqail, A.; Elmaghraby, M.A.; Elkady, O.A. Microstructure, hardness, and tribology properties of the (Cu/MoS2)/graphene nanocomposite via the electroless deposition and powder metallurgy technique. J. Compos. Mater. 2020, 54–23, 3435–3446. [Google Scholar] [CrossRef]
- Sadoun, A.M.; Mohammed, M.M.; Elsayed, E.M.; Meselhy, A.F.; El-Kady, O. A Effect of nano Al2O3 coated Ag addition on the corrosion resistance and electrochemical behavior of Cu-Al2O3 nanocomposites. J. Mater. Res. Technol. 2020, 9–3, 4485–4493. [Google Scholar] [CrossRef]














| Alloys | Cu wt.% | Zn wt.% | GNSs wt.% |
|---|---|---|---|
| Cu94Zn5GNSs1 | 94 | 5 | 1 |
| Cu89Zn10GNSs1 | 89 | 10 | 1 |
| Cu84Zn15GNSs1 | 84 | 15 | 1 |
| Cu79Zn20GNSs1 | 79 | 20 | 1 |
| Sample | Process Control Agent (PCA) | Relative Density |
|---|---|---|
| Cu-1 wt.% GNSs | Hexane | 86.35 |
| Cu-1 wt.% GNSs | Methanol | 76.32 |
| Alloys | Relative Density % |
|---|---|
| Cu94Zn5GNSs1 | 89.90 |
| Cu89Zn10GNSs1 | 90.64 |
| Cu84Zn15GNSs1 | 87.41 |
| Cu79Zn20GNSs1 | 85.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamed, A.T.; Mosa, E.S.; Mahdy, A.; El-Batanony, I.G.; A. Elkady, O. Preparation and Evaluation of Cu-Zn-GNSs Nanocomposite Manufactured by Powder Metallurgy. Crystals 2021, 11, 1449. https://doi.org/10.3390/cryst11121449
Hamed AT, Mosa ES, Mahdy A, El-Batanony IG, A. Elkady O. Preparation and Evaluation of Cu-Zn-GNSs Nanocomposite Manufactured by Powder Metallurgy. Crystals. 2021; 11(12):1449. https://doi.org/10.3390/cryst11121449
Chicago/Turabian StyleHamed, A. T., E. S. Mosa, Amir Mahdy, Ismail G. El-Batanony, and Omayma A. Elkady. 2021. "Preparation and Evaluation of Cu-Zn-GNSs Nanocomposite Manufactured by Powder Metallurgy" Crystals 11, no. 12: 1449. https://doi.org/10.3390/cryst11121449