A Multifaceted Kinetic Model for the Thermal Decomposition of Calcium Carbonate
Abstract
:1. Introduction
2. Kinetic Model
3. Experimental Details
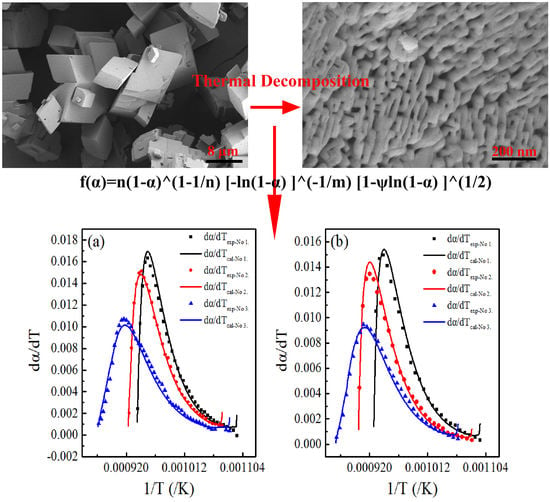
4. Results and Discussion
4.1. Comparison with Other Models
4.2. Calculation of Activation Energy
4.3. Rationality of the Multifaceted Model
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Momenzadeh, L.; Moghtaderi, B.; Buzzi, O.; Liu, X.; Sloan, S.W.; Murch, G.E. The thermal conductivity decomposition of calcite calculated by molecular dynamics simulation. Comput. Mater. Sci. 2018, 141, 170–179. [Google Scholar] [CrossRef]
- Du, H.; Steinacher, M.; Borca, C.; Huthwelker, T.; Murello, A.; Stellacci, F.; Amstad, E. Amorphous CaCO3: Influence of the Formation Time on Its Degree of Hydration and Stability. J. Am. Chem. Soc. 2018, 140, 14289–14299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, H.; Alfredsson, V.; Tropsch, J.; Ettl, R.; Nylander, T. Formation of CaCO3 deposits on hard surfaces--effect of bulk solution conditions and surface properties. ACS Appl. Mater. Interfaces 2013, 5, 4035–4045. [Google Scholar] [CrossRef] [PubMed]
- Valverde, J.M.; Sanchez-Jimenez, P.E.; Perez-Maqueda, L.A. Limestone Calcination Nearby Equilibrium: Kinetics, CaO Crystal Structure, Sintering and Reactivity. J. Phys. Chem. C 2015, 119, 1623–1641. [Google Scholar] [CrossRef] [Green Version]
- Escardino, A.; Garcia-Ten, J.; Feliu, C.; Gozalbo, A. Calcium carbonate thermal decomposition in white-body wall tile during firing. II. Influence of body thickness and calcite content. Ceram. Int. 2012, 38, 3141–3147. [Google Scholar] [CrossRef]
- Escardino, A.; García-Ten, J.; Feliu, C.; Moreno, A. Calcium carbonate thermal decomposition in white-body wall tile during firing. I. Kinetic study. J. Eur. Ceram. Soc. 2010, 30, 1989–2001. [Google Scholar] [CrossRef]
- Bennadji, H.; Khachatryan, L.; Lomnicki, S.M. Kinetic Modeling of Cellulose Fractional Pyrolysis. Energy Fuels 2018, 32, 3436–3446. [Google Scholar] [CrossRef]
- Sánchez-Jiménez, P.E.; Perejón, A.; Pérez-Maqueda, L.A.; Criado, J.M. New Insights on the Kinetic Analysis of Isothermal Data: The Independence of the Activation Energy from the Assumed Kinetic Model. Energy Fuels 2014, 29, 392–397. [Google Scholar] [CrossRef]
- Tan, G.; Wang, Q.; Zheng, H.; Zhao, W.; Zhang, S.; Liu, Z. Concept of variable activation energy and its validity in nonisothermal kinetics. J. Phys. Chem. A 2011, 115, 5517–5524. [Google Scholar] [CrossRef]
- Westbrook, C.K.; Mehl, M.; Pitz, W.J.; Kukkadapu, G.; Wagnon, S.; Zhang, K. Multi-fuel surrogate chemical kinetic mechanisms for real world applications. Phys. Chem. Chem. Phys. 2018, 20, 10588–10606. [Google Scholar] [CrossRef]
- Zhou, D.G.; David, J.W. Model Dependence of the Activation Energy Derived from Nonisothermal Kinetic Data. J. Phys. Chem. A 2004, 108, 19. [Google Scholar] [CrossRef]
- Satterfield, C.N.; Feakes, F. Kinetics of the thermal decomposition of calcium carbonate. AICHE J. 1959, 115–122. [Google Scholar] [CrossRef]
- Maitra, S.; Choudhury, A.; Das, H.S.; Pramanik, M.J. Effect of compaction on the kinetics of thermal decomposition of dolomite under non-isothermal condition. J. Mater. Sci. 2005, 40, 4749–4751. [Google Scholar] [CrossRef]
- Rao, T.R. Kinetics of calcium carbonate decomposition. Chem. Eng. Technol. 1996, 19, 373–377. [Google Scholar] [CrossRef]
- Criado, J.M.; Ortega, A. A study of the influence of particle size on the thermal decomposition of CaCO3 by means of constant rate thermal analysis. Thermochim. Acta 1992, 195, 163–167. [Google Scholar] [CrossRef]
- Chinyama, M.P.M.; Lockwood, F.C.; Yousif, S.Y.A.; Kandamby, N. Modelling of calcium carbonate decomposition in cement plant precalciners. J. Energy Inst. 2008, 81, 19–24. [Google Scholar] [CrossRef]
- Ingraham, T.R.; Marier, P. Kinetic studies on the thermal decomposition of calcium carbonate. Can. J. Chem. Eng. 1963, 41, 170–173. [Google Scholar] [CrossRef]
- Calvo, E.G.; Arranz, M.A.; Letón, P. Effects of impurities in the kinetics of calcite decomposition. Thermochim. Acta 1990, 170, 7–11. [Google Scholar] [CrossRef]
- Beruto, D.; Searcy, A.W. Use of the Langmuir method for kinetic studies of decomposition reactions: Calcite (CaCO3). J. Chem. Soc. Faraday Trans. Phys. Chem. Condens. Phases 1974, 70, 2145. [Google Scholar] [CrossRef]
- Beruto, D.T.; Vecchiattini, R.; Giordani, M. Solid products and rate-limiting step in the thermal half decomposition of natural dolomite in a CO2 (g) atmosphere. Thermochim. Acta 2003, 405, 183–194. [Google Scholar] [CrossRef]
- Bouineau, V.; Pijolat, M.; Soustelle, M. Characterisation of the chemical reactivity of a CaCO3 powder for its decomposition. J. Eur. Ceram. Soc. 1998, 18, 1319–1324. [Google Scholar] [CrossRef]
- Chen, H.; Liu, N. Application of Non-Arrhenius Equations in Interpreting Calcium Carbonate Decomposition Kinetics: Revisited. J. Am. Ceram. Soc. 2010, 93, 548–553. [Google Scholar] [CrossRef]
- Brown, D.D.; Galwey, M.E. Comprehensive Chemical Kinetics; Elsevier Scientific Publishing Company: Amsterdam, The Netherlands, 1980. [Google Scholar]
- Georgieva, V.; Vlaev, L.; Gyurova, K. Non-Isothermal Degradation Kinetics of CaCO3 from Different Origin. J. Chem. 2013, 2013, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Maitra, S.; Bandyopadhyay, N.; Das, S.; Pal, A.J.; Pramanik, J. Non-Isothermal Decomposition Kinetics of Alkaline Earth Metal Carbonates. J. Am. Ceram. Soc. 2007, 90, 1299–1303. [Google Scholar] [CrossRef]
- Beruto, D.; Searcy, A.W. Calcium oxides of high reactivity. Nature 1976, 263, 221–222. [Google Scholar] [CrossRef]
- Bertrand, G. Kinetics of endothermic decomposition reactions. 2. Effect of the solid and gaseous products. Comments. J. Phys. Chem. 1978, 82, 2536–2537. [Google Scholar] [CrossRef]
- Mulokozi, A.M.; Lugwisha, E. New aspects of the decomposition kinetics of calcite. Thermochim. Acta 1992, 194, 375–383. [Google Scholar] [CrossRef]
- Zhang, J.-L.; Wang, G.-W.; Shao, J.-G.; Zuo, H.-B. A Modified Random Pore Model for the Kinetics of Char Gasification. BioResources 2014, 9, 3497–3507. [Google Scholar] [CrossRef]
- Wang, G.W.; Zhang, J.L.; Shao, J.G.; Liu, Z.J.; Wang, H.Y.; Li, X.Y.; Zhang, P.C.; Geng, W.W.; Zhang, G.H. Experimental and modeling studies on CO2 gasification of biomass chars. Energy 2016, 114, 143–154. [Google Scholar] [CrossRef] [Green Version]
- Lahijani, P.; Zainal, Z.A.; Mohamed, A.R.; Mohammadi, M. Co-gasification of tire and biomass for enhancement of tire-char reactivity in CO2 gasification process. Bioresour. Technol. 2013, 138, 124–130. [Google Scholar] [CrossRef]
- Sharp, J.H.; Wentworth, S.A. Kinetic analysis of thermogravimetric data. Anal. Chem. 2002, 41, 2060–2062. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Wang, H.; Chen, X.; Dai, W.; Fu, X. The mechanism of photodriven oxidation of CO over anatase and rutile TiO2 investigated from the hydrogen adsorption behavior. Appl. Catal. B Environ. 2020, 277, 119169. [Google Scholar] [CrossRef]
- Šesták, J. Diagnostic limits of phenomenological kinetic models introducing the accommodation function. J. Therm. Anal. 1990, 36, 1997–2007. [Google Scholar] [CrossRef]
- Sestak, J.; Malek, J. Diagnostic limits of phenomenological models of heterogeneous reactions and thermal analysis kinetics. Solid State Ionics 1993, 65, 245–254. [Google Scholar] [CrossRef]
- Gorbachev, V.M. A method for determining the starting temperature of the thermal effect. J. Therm. Anal. 1980, 19, 377–380. [Google Scholar] [CrossRef]
- Sanders, J.P.; Gallagher, P.K. Kinetic analyses using simultaneous TG/DSC measurements. J. Therm. Anal. Calorim. 2005, 82, 659–664. [Google Scholar] [CrossRef]
- Thomas, J.M.; Renshaw, G.D. Influence of dislocations on the thermal decomposition of calcium carbonate. J. Chem. Soc. A Inorg. Phys. Theor. 1967, 2058–2061. [Google Scholar] [CrossRef]
- Dollimore, D.; Evans, T.A.; Lee, Y.F.; Pee, G.P.; Wilburn, F.W. The significance of the onset and final temperatures in the kinetic analysis of TG curves. Thermochim. Acta 1992, 196, 255–265. [Google Scholar] [CrossRef]
- Dollimore, D.; Tong, P.; Alexander, K.S. The kinetic interpretation of the decomposition of calcium carbonate by use of relationships other than the Arrhenius equation. Thermochim. Acta 1996, 283, 13–27. [Google Scholar] [CrossRef]
- Maciejewski, M. Computational aspects of kinetic analysis. Thermochimica Acta. 2000, 355, 145–154. [Google Scholar] [CrossRef]
- Britton, H.T.S.; Gregg, S.J.; Winsor, G.W. The calcination of dolomite. Part I—The kinetics of the thermal decomposition of calcite and of magnesite. Trans. Faraday Soc. 1952, 48, 63–69. [Google Scholar] [CrossRef]
- Hills, A.W.D. The mechanism of the thermal decomposition of calcium carbonate. Chem. Eng. Sci. 1968, 23, 297–320. [Google Scholar] [CrossRef]
- Deutsch, Y.; Heller-Kallai, L. Decarbonation and recarbonation of calcites heated m CO2. Thermochim. Acta 1991, 182, 77–89. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Ruiz-Agudo, E.; Luque, A.; Rodriguez-Navarro, A.B.; Ortega-Huertas, M. Thermal decomposition of calcite: Mechanisms of formation and textural evolution of CaO nanocrystals. Am. Mineral. 2009, 94, 578–593. [Google Scholar] [CrossRef]
- Escardino, A.; Garcia-Ten, J.; Feliu, C. Kinetic study of calcite particle (powder) thermal decomposition: Part I. J. Eur. Ceram. Soc. 2008, 28, 3011–3020. [Google Scholar] [CrossRef]
- Fei, H.; Hu, S.; Xiang, J.; Sun, L.; Zhang, A.; Fu, P.; Wang, B.; Chen, G. Modified Random Pore Model Study on Coal Char Reactions under O2/CO2 Atmosphere. In Proceedings of the 2010 Asia-Pacific Power and Energy Engineering Conference, Chengdu, China, 28–31 March 2010. [Google Scholar] [CrossRef]
- Cai, J.; Wang, S.; Kuang, C. A Modified Random Pore Model for Carbonation Reaction of CaO-based Limestone with CO2 in Different Calcination-carbonation Cycles. Energy Procedia 2017, 105, 1924–1931. [Google Scholar] [CrossRef]








| Sample | Models | E (kJ/mol) | A (s−1) | |
|---|---|---|---|---|
| No.1 | A1 | 325.43 | 2.28 × 1016 | 21.68 |
| R2 | 235.78 | 1.13 × 1011 | 5.36 | |
| R3 | 265.66 | 3.50 × 1012 | 9.74 | |
| D2 | 513.91 | 3.50 × 1025 | 26.01 | |
| D4 | 546.73 | 5.27 × 1026 | 20.10 | |
| 178.16 | 1.58 × 107 | 3.27 | ||
| No.2 | A1 | 318.90 | 5.92 × 1015 | 20.03 |
| R2 | 234.06 | 6.16 × 1010 | 3.73 | |
| R3 | 262.34 | 1.49 × 1012 | 8.66 | |
| D2 | 516.18 | 2.04 × 1025 | 27.19 | |
| D4 | 547.05 | 2.28 × 1026 | 21.33 | |
| 177.63 | 1.09 × 107 | 1.58 | ||
| No.3 | A1 | 243.83 | 4.45 × 1011 | 13.37 |
| R2 | 144.93 | 8.67 × 105 | 8.21 | |
| R3 | 177.90 | 3.67 × 107 | 3.07 | |
| D2 | 346.07 | 1.27 × 1016 | 81.41 | |
| D4 | 382.44 | 2.75 × 1017 | 66.55 | |
| 140.27 | 5.35 × 104 | 3.43 |
| Sample | E (kJ/mol) | A (s−1) | |
|---|---|---|---|
| No.1 ( = 2, = 4, = 50) | 178.16 | 1.58 × 107 | 3.27 |
| No.2 ( = 2, = 4, = 50) | 177.63 | 1.09 × 107 | 1.58 |
| No.3 ( = 4, = 4, = 50) | 140.27 | 5.35 × 104 | 3.43 |
| Sample | Heating Rate | E (kJ/mol) | A (s−1) | |
|---|---|---|---|---|
| No.1 ( = 2, = 4, = 50) | 10 K/min | 178.56 | 1.80 × 107 | 3.24 |
| 15 K/min | 178.46 | 2.00 × 107 | 4.92 | |
| No.2 ( = 2, = 4, = 50) | 10 K/min | 177.60 | 1.15 × 107 | 1.94 |
| 15 K/min | 177.66 | 1.04 × 107 | 5.92 | |
| No.3 ( = 4, = 4, = 50) | 10 K/min | 141.46 | 5.60 × 104 | 2.22 |
| 15 K/min | 140.97 | 6.05 × 104 | 3.19 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, J.; Huang, J.; Tao, L.; Li, Z.; Wang, Q. A Multifaceted Kinetic Model for the Thermal Decomposition of Calcium Carbonate. Crystals 2020, 10, 849. https://doi.org/10.3390/cryst10090849
Zheng J, Huang J, Tao L, Li Z, Wang Q. A Multifaceted Kinetic Model for the Thermal Decomposition of Calcium Carbonate. Crystals. 2020; 10(9):849. https://doi.org/10.3390/cryst10090849
Chicago/Turabian StyleZheng, Jingxue, Junchen Huang, Lin Tao, Zhi Li, and Qi Wang. 2020. "A Multifaceted Kinetic Model for the Thermal Decomposition of Calcium Carbonate" Crystals 10, no. 9: 849. https://doi.org/10.3390/cryst10090849