Synthesis and Characterization of Photoluminescence Liquid Crystals Based on Flexible Chain-Bearing Pentafluorinated Bistolanes
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
2.2. Materials
2.3. Typical Synthesis of R-4b
2.4. Typical Synthesis of R-5b
2.5. Typical Synthesis of R-2b
2.6. Computations
2.7. Phase Transition Behavior
2.8. Photophysical Behavior
3. Results and Discussion
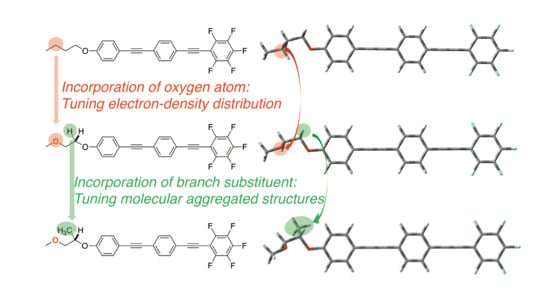
3.1. Theoretical Molecular Design and Synthesis
3.2. Phase Transition Behavior
3.3. Photophysical Behavior
3.3.1. Photophysical Behavior in Solution
3.3.2. Photophysical Behavior in Molecular Aggregated Phases
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Singh, H.; Tiwari, K.; Tiwari, R.; Pramanik, S.K.; Das, A. Small molecules as fluorescent probes for monitoring intracellular enzymatic transformations. Chem. Rev. 2019, 119, 11718–11760. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. AIEgens for biological process for monitoring and disease theranostics. Biomaterials 2017, 146, 115–135. [Google Scholar] [CrossRef] [PubMed]
- Hemmer, E.; Acosta-Mora, P.; Méndez-Ramos, J.; Fischer, S. Optical nanoprobes for biomedical applications: Shining a light on upconverting and near-infrared emitting nanoparticles for imaging, thermal sensing, and photodynamic therapy. J. Mater. Chem. B 2017, 5, 4365–4392. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, F.; Hyun, J.Y.; Wei, T.; Qiang, J.; Ren, X.; Shin, I.; Yoon, J. Recent progress in the development of fluorescent, luminescent and colorimetric probes for detection of reactive oxygen and nitrogen species. Chem. Soc. Rev. 2016, 45, 2976–3016. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, Q.; Feng, W.; Li, F. Luminescent chemodosimeters for bioimaging. Chem. Rev. 2013, 113, 192–270. [Google Scholar] [CrossRef]
- Yersin, H. (Ed.) Highly Efficient OLEDs: Materials Based on Thermally Activated Delayed Fluorescence; Wiley-VCH: Weinheim, Germany, 2018. [Google Scholar]
- Bui, T.-T.; Goubard, F.; Ibrahim-Ouali, M.; Gigmes, D.; Dumur, F. Recent advances on organic blue thermally activated delayed fluorescence (TADF) emitters for organic light-emitting diodes (OLEDs). Beilstein J. Org. Chem. 2018, 14, 282–308. [Google Scholar] [CrossRef] [Green Version]
- Jhulki, S.; Moorthy, J.N. Small molecular hole-transporting materials (HTMs) in organic light-emitting diodes (OLEDs): Structural diversity and classification. J. Mater. Chem. C 2018, 6, 8280–8325. [Google Scholar] [CrossRef]
- Gaspar, D.J.; Polikarpov, E. (Eds.) OLED Fundamentals: Materials, Devices, and Processing of Organic Light-Emitting Diodes; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Ostroverkhova, O. Organic optoelectronic materials: Mechanisms and applications. Chem. Rev. 2016, 116, 13279–13412. [Google Scholar] [CrossRef]
- Birks, J.B. (Ed.) Photophysics of Aromatic Molecules; Wiley: London, UK, 1970. [Google Scholar]
- Thomas, S.W.; Joly, G.D.; Swager, T.M. Chemical sensors based on amplifying fluorescent conjugated polymers. Chem. Rev. 2007, 107, 1339–1386. [Google Scholar] [CrossRef]
- Li, H.; Li, B.S.; Tang, B.Z. Molecular design, circularly polarized luminescence, and helical self-assembly of chiral aggregation-induced emission molecules. Chem. Asian J. 2019, 14, 674–688. [Google Scholar] [CrossRef]
- He, Z.; Ke, C.; Tang, B.Z. Journey of aggregation-induced emission research. ACS Omega 2018, 3, 3267–3277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-induced emission: Together we shine, united we soar! Chem. Rev. 2015, 21, 11718–11940. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; He, B.; Tang, B.Z. Aggregation-induces emission of siloles. Chem. Sci. 2015, 6, 5347–5365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumon, T.; Hashishita, S.; Kida, T.; Yamada, S.; Ishihara, T.; Konno, T. Gram-scale preparation of negative-type liquid crystals with a CF2CF2-carbocycle unit via an improved short-step synthetic protocol. Beilstein J. Org. Chem. 2018, 14, 148–154. [Google Scholar] [CrossRef] [Green Version]
- Yamada, S.; Tamamoto, K.; Kida, T.; Asai, T.; Ishihara, T.; Konno, T. Rational design and synthesis of a novel laterally-tetrafluorinated tricyclic mesogen with large negative dielectric anisotropy. Org. Biomol. Chem. 2017, 15, 9442–9454. [Google Scholar] [CrossRef]
- Yamada, S.; Hashishita, S.; Asai, T.; Ishiahra, T.; Konno, T. Design, synthesis and evaluation of new fluorinated liquid crystals bearing CF2CF2 fragment with negative dielectric anisotropy. Org. Biomol. Chem. 2017, 15, 1495–1509. [Google Scholar] [CrossRef]
- Morita, M.; Yamada, S.; Konno, T. Fluorine-induced emission enhancement of tolanes via formation of tight molecular aggregates. New J. Chem. 2020, 44, 6704–6708. [Google Scholar] [CrossRef]
- Yamada, S.; Nishizawa, A.; Morita, M.; Hosokai, T.; Okabayashi, Y.; Agou, T.; Hosoya, T.; Kubota, T.; Konno, T. Synthesis and characterization of bent fluorine-containing donor-π-acceptor molecules as intense luminophores with large Stokes shifts. Org. Biomol. Chem. 2019, 17, 6911–6919. [Google Scholar] [CrossRef]
- Yamada, S.; Morita, M.; Konno, T. Multi-color photoluminescence induced by electron-density distribution of fluorinated bistolane derivatives. J. Fluor. Chem. 2017, 202, 54–64. [Google Scholar] [CrossRef]
- Yamada, S.; Mitsuda, A.; Adachi, K.; Hara, M.; Konno, T. Development of light-emitting liquid-crystalline polymers with a pentafluorinated bistolane-based luminophore. New J. Chem. 2020, 44, 5684–5691. [Google Scholar] [CrossRef]
- Morita, M.; Yamada, S.; Agou, T.; Kubota, T.; Konno, T. Luminescence tuning of fluorinated bistolanes via electronic or aggregated-structure control. Appl. Sci. 2019, 9, 1905. [Google Scholar] [CrossRef] [Green Version]
- Yamada, S.; Miyano, K.; Agou, T.; Kubota, T.; Konno, T. 2-Chloroalkoxy-substituted pentafluorinated bistolanes as novel light-emitting liquid crystals. Crystals 2019, 9, 195. [Google Scholar] [CrossRef] [Green Version]
- Yamada, S.; Miyano, K.; Konno, T.; Agou, T.; Kubota, T.; Hosokai, T. Fluorine-containing bistolanes as light-emitting liquid crystalline molecules. Org. Biomol. Chem. 2017, 15, 5949–5958. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision B.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Chan, B.; Gilbert, A.T.B.; Gill, P.M.W.; Radom, L. Performance of density functional theory procedures for the calculation of proton-exchange barriers: Unusual behavior of M06-type functionals. J. Chem. Theory Comput. 2014, 10, 3777–3783. [Google Scholar] [CrossRef] [PubMed]
- Andzelm, J.; Kölmel, C.; Klamt, A. Incorporation of solvent effects into density functional calculations of molecular energies and geometries. J. Chem. Phys. 1995, 103, 9312–9320. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M. Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef]
- Tamura, R.; Takahashi, H.; Ushio, T. New enantiomeric resolution phenomenon of racemic crystals: Preferential enrichment. J. Synth. Org. Chem. Jpn. 1998, 56, 22–33. [Google Scholar] [CrossRef] [Green Version]
- Gelebart, A.H.; Mulder, D.J.; Varga, M.; Konya, A.; Ghislaine, V.; Meijer, E.W.; Selinger, R.L.B.; Broer, D.J. Making waves in a photoactive polymer film. Nature 2017, 546, 632–636. [Google Scholar] [CrossRef] [Green Version]
- Ravi, M.; Samanta, A.; Radhakrishnan, T. Excited state dipole moments from an efficient analysis of solvatochromic Stokes shift data. J. Phys. Chem. 1994, 98, 9133–9136. [Google Scholar] [CrossRef]
- Yao, H.; Okada, T.; Mataga, N. Solvation-induced charge separation in the excited state of composite systems with identical halves and intramolecular excimer formation by recombination: Picosecond laser photolysis studies on 1,2-dianthrylethanes. J. Phys. Chem. 1989, 93, 7388–7394. [Google Scholar] [CrossRef]
- Mataga, N.; Kaifu, Y.; Koizumi, M. The solvent effect on fluorescence spectrum. Change of solute-solvent interaction during the lifetime of excited solute molecule. Bull. Chem. Soc. Jpn. 1955, 28, 690–691. [Google Scholar] [CrossRef] [Green Version]
- Iida, A.; Yamaguchi, S. Intense solid-state blue emission with a small Stokes’ shift: π-Stacking protection of the diphenylanthracene skeleton. Chem. Commun. 2009, 3002–3004. [Google Scholar] [CrossRef]








| Molecule | μ|| (D) 2 | HOMO/LUMO 3 (eV) | Theoretical Transition Energy (nm) 4 | f5 |
|---|---|---|---|---|
| 1 | 5.28 | −7.05/−1.59 | 335 nm | 2.298 |
| 2a | 6.21 | −7.06/−1.59 | 334 nm | 2.294 |
| S-2b | 6.73 | −7.04/−1.59 | 335 nm | 2.305 |
| Molecule | Phase Transition Sequence (Enthalpy [kJ mol−1]) 2 | |
|---|---|---|
| 2a | Heating | Cr 134 (27.3) N 212 (0.58) Iso |
| Cooling | Cr 127 (−26.2) N 212 (−0.49) Iso | |
| rac-2b | Heating | Cr1 61 (1.21) Cr2 82 (−5.64) Cr3 97 (28.9) N 129 (0.23) Iso |
| Cooling | Cr1 54 (−0.79) Cr2 79 (−4.40) Cr3 87 (−17.5) N 129 (−0.29) Iso | |
| R-2b | Heating | Cr 102 (117.1) N* 130 (0.88) Iso |
| Cooling | Cr 85 (−111.9) N* 130 (−0.81) Iso | |
| S-2b | Heating | Cr 102 (32.9) N* 130 (0.35) Iso |
| Cooling | Cr 85 (−31.7) N* 130 (−0.26) Iso |
| Molecule | Solvent (ε) 1 | λabs [nm] 2 (ε, [L mol–1 cm–1]) 3 | λPL [nm] 4 (ΦPL) 5 | CIE Coordinate (x, y) |
|---|---|---|---|---|
| 2a | CH2Cl2 (8.93) | 332 (64300) | 406 (0.91) | (0.16, 0.19) |
| rac-2b | CH2Cl2 (8.93) | 333 (70000) | 412 (0.70) | (0.15, 0.11) |
| R-2b | CH2Cl2 (8.93) | 333 (62900) | 412 (0.88) | (0.16, 0.10) |
| S-2b | CH2Cl2 (8.93) | 332 (82700) | 412 (0.99) | (0.16, 0.09) |
| Toluene (2.38) | 335 (58100) | 381, 397sh (0.79) | (0.16, 0.04) | |
| CHCl3 (4.81) | 333 (57300) | 393, 404sh (0.93) | (0.16, 0.04) | |
| THF (7.58) | 333 (53500) | 416 (0.98) | (0.16, 0.06) | |
| MeCN (35.9) | 329 (56200) | 434 (0.92) | (0.16, 0.12) | |
| DMF (36.7) | 333 (41700) | 443 (0.71) | (0.17, 0.15) |
| Molecule | Phase 1 | λPL [nm] 2 (ΦPL) 3 | CIE Coordinate (x, y) |
|---|---|---|---|
| 2a | Cr | 465 (0.39) | (0.16, 0.19) |
| N | 412sh, 442 (0.17) | (0.16, 0.12) | |
| rac-2b | Cr | 449 (0.70) | (0.15, 0.11) |
| N | 434 (0.58) | (0.16, 0.10) | |
| R-2b | Cr | 439 (0.68) | (0.16, 0.10) |
| N* | 436 (0.41) | (0.16, 0.10) | |
| S-2b | Cr | 432 (0.74) | (0.16, 0.08) |
| N* | 435 (0.40) | (0.16, 0.10) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamada, S.; Sato, M.; Konno, T. Synthesis and Characterization of Photoluminescence Liquid Crystals Based on Flexible Chain-Bearing Pentafluorinated Bistolanes. Crystals 2020, 10, 603. https://doi.org/10.3390/cryst10070603
Yamada S, Sato M, Konno T. Synthesis and Characterization of Photoluminescence Liquid Crystals Based on Flexible Chain-Bearing Pentafluorinated Bistolanes. Crystals. 2020; 10(7):603. https://doi.org/10.3390/cryst10070603
Chicago/Turabian StyleYamada, Shigeyuki, Masaya Sato, and Tsutomu Konno. 2020. "Synthesis and Characterization of Photoluminescence Liquid Crystals Based on Flexible Chain-Bearing Pentafluorinated Bistolanes" Crystals 10, no. 7: 603. https://doi.org/10.3390/cryst10070603