1. Introduction
The MgO-TiO
2 ceramic system includes three stoichiometric magnesium titanate phases: Mg
2TiO
4 (qandilite), MgTiO
3 (geikielite) and MgTi
2O
5 (karrooite) [
1]. Their crystal structures have been determined by X-ray diffraction and neutron diffraction [
2,
3]. The phases are:
- (a)
Geikielite, MgTiO3, is rhombohedral of ilmenite type, space group R-3 (148), Pearson Symbol hR10.0; formed at temperatures above 600 °C, is stable from room temperature to its melting point;
- (b)
Qandilite, Mg2TiO4, cubic of inverse spinel type, space group Fd-3m (227), Pearson Symbol cF56.0, formed at above 1150 °C;
- (c)
Karrooite, MgTi2O5, orthorhombic of pseudo-brookite type, space group Cmcm (63), Pearson Symbol oC32.0 formed above 500 °C.
High-temperature X-ray diffraction (HT-XRD) studies have been published for all three stoichiometric Mg titanates prepared by the sol-gel technique during their transformation from the xerogel precursor to their final oxide form for a range of temperatures between 700 and 1300 °C [
4]. High-temperature neutron diffraction (HT-ND) studies have been made on Mg
2TiO
4 synthesized by the solid-state reaction at temperatures between 90 to 1400 °C [
5]. In this study, there was agreement between the lattice parameter of the HT-XRD and HT-ND data in the temperature range between 800 and 1300 °C [
6]. Importantly, the in-situ HT-XRD studies of MgTiO
3 during the firing of xerogel powders between 700 and 1300 °C yielded linear thermal expansion coefficients (TECs).
There are two additional publications with ND-XRD data for MgTiO
3, which by contrast, showed non-linear TECs [
7,
8]. Both publications are for the same sample -Kar2-, studied where the MgTi
2O
5 (karrooite) phase was the major component and the MgTiO
3 (geikielite) and TiO
2 (rutile) phases were the minor components (by mass). In these publications, the phases were obtained by the solid-state reaction between MgCO
3 and TiO
2 fine powders, and the HT-ND data [
7,
8] were collected on sample Kar2 between 23 and 1305 °C.
Further examination of the HT diffraction works on geikielite showed that there is a significant gap in the lattice parameters between the HT-XRD data received from sol-gel product [
4], and HT-ND data received from geikielite using the solid state reaction in that the lattice parameters of the sol-gel product were slightly lower. A key difference in the collection of the data was that the sol-gel data were obtained using a different sample at each temperature after 1 h firing, and the solid-state version was a single sample for all temperatures. In order to determine if the gap originated from the experimental methods or inherent differences in the synthesis (sol-gel vs. solid state), it was decided to investigate a single geikielite sample determining lattice parameters between RT up to 900 °C.
In this work, we complete the HT crystallographic data of sol-gel product of MgTiO3 using HT-XRD measurements of MgTiO3 made by sol-gel technique between 25 and 900 °C and provide insight to the differences in TEC behavior.
2. Materials and Methods
2.1. Synthesis
The magnesium titanates were prepared by the sol-gel method using metalorganic precursors: diethyl ethoxymagnesiomalonate, prepared by metallation of diethyl malonate (1), and titanium(IV) tetra-tert-butoxide (Aldrich), dissolved in anhydrous 2-propanol. The hydrolysis step was carried out at nearly room temperature, using a stream of hot air (about 100 °C) containing water vapor (superheated steam), during about 3 hours, to ensure total hydrolysis. The precipitated solid was filtered, washed with 2-propanol and left to dry in air for several days, until a constant weight was obtained. After analysis of the Mg and Ti content, this solid served as starting material for thermal treatment.
The powders were initially fired at 600 °C for 3 h. After the initial treatment, the sample with Mg:Ti 1.1:1 found as a single geikielite phase, where a = 5.0537 (3) Å; c = 13.897 (5) Å; atomic positions: z(Mg) = 0.3563 (2); z(Ti) = 0.1445 (2); x(O) = 0.315(1); y(O) = 0.0225(1); z(O) = 0.247(1). However, the expected Mg:Ti ratio was confirmed by inductively coupled plasma (ICP). The amount of impurities was below 10 ppm. Additional treatments were made at 1200 °C for 5 h in order to obtain well-crystallized powders. After the 1200 °C treatment, the sample was found with 95% geikielite and 5% qandilite. It was designated as “GQ” (major phase geikielite and minor phase qandilite) and will be referred to as such throughout the manuscript. The qandilite within GQ served as the internal standard.
2.2. RT XRD Measurements
The samples for HT-XRD studies were characterized by a Rigaku powder X-Ray Diffractometer. Data were collected in the conventional Bragg–Brentano configuration (theta/2theta) by means of Cu K
α radiation at 40 kV and 30 mA. The K
β was filtered out by graphite monochromator attached to the detector. Phase characterization from XRD data was made by using public domain FullProf/WinPlotter software [
9]).
2.3. HT XRD Measurements
X-ray diffraction was performed on a Bruker D8 Advance in Bragg–Brentano geometry using an X-ray source with a Cu anode having a Kα1 emission wavelength of 1.5406 angstroms. Samples were placed in an Anton Paar XRK-900 high-temperature reaction chamber using a Macor sample stage. The influence of the thermal expansion of the stage was measured by calibrating the stage height as a function of temperature using an alignment slit. The temperature of the sample was controlled using and Anton Paar TCU 750 controller by mounting a K-type thermocouple in the sample holder adjacent to the sample. A linear PSD detector (LYNXEYE XE-T) was used with an opening of 2.94 degrees. The diffraction pattern was recorded from two-theta of 10 degrees until 120 degrees using a coupled theta/two-theta scan type. Data points were acquired in increments of 0.02 degrees with an acquisition time of 0.25 s. Lattice parameters were fitted using TOPAS software with a TCHZ function. The refined parameters included the lattice parameters, sample displacement and zero error. The line position and effect of instrumental broadening and asymmetry were calibrated by SRM 660c LaB6.
The diffractograms were also analyzed by the program Powder-Cell [
10]. In this step, the phases were easily identified, and the unit cells were verified for the thermal expansion. Grain shape and size was assessed by HR-SEM.
2.4. Thermal Expansion Methodology
It is essential to know the dimensions of ceramic materials as function of temperatures in order to calculate the dimensions of objects working at elevated temperatures and to evaluate thermal stresses during temperature changes. For practical reasons it is suggested to define the relative dimension change of a material by Equation (1):
where
L(
T) is the size of one dimension at temperature
T,
L0 is the size of the same dimension at ambient temperature,
T is the working temperature and
T0 is the ambient temperature (usually the ambient temperature is 25 °C).
In the case of linear thermal expansion, Equation (1) becomes
where α is the linear thermal expansion coefficient. For a general case it is possible to define
or
where
Neglecting the contribution of enhanced vacancy formation at higher temperatures to the size of a sample of matter, the thermal expansion of a periodical crystalline matter can be modeled by measuring its lattice parameters as function of temperature. By using HT diffraction, each lattice parameter,
Aj, can be obtained directly from the measurement as
In this case, the non-linear thermal coefficients will be given as
From Equation (3) it is then possible to define an overall thermal expansion coefficient along a crystal axis
Aj as
Similarly, for unit cell volume
V we define the volumetric thermal expansion as
It should be noted that this methodology is valid only where there is no phase transformation or significant crystal structure change in the range of measurements.
4. Discussion
Figure 2 shows that the lattice parameters versus temperature of the reference material, Mg
2TiO
4, are in excellent agreement with the published HT-ND data [
5].
Figure 4 shows a comparison between calculated lattice parameters versus temperature derived from Equations (9) and (10) showing complete overlapping of the lattice parameters of the reference material (Mg
2TiO
4) (Equation (9)) and published HT-ND study of Mg
2TiO
4 [
5].
A comparison of
Table 5 and
Table 6 shows that in MgTiO
3 there was no significant change in the atomic positions along the experimental temperature range (25–890 °C).
There was excellent agreement with present and previous HT-XRD data of a sol-gel MgTiO
3 product after 1 h firing [
4] (
Figure 3). The agreement with former HT-XRD on sol-gel products should be appreciated because, in contrast to usual TEC investigations, which are done on a single sample, it was done with different sample at each temperature. In order to eliminate the experimental scattering, the calculated lattice parameters versus temperature were plotted.
Figure 5 shows that there was fair agreement between the lattice parameters versus temperature, between RT and 1300 °C, of all the sol-gel products of MgTiO
3 studied in HT-XRD data (present work and Reference [
4]) in comparison with published results of minor MgTiO
3 phase in reference [
7,
8].
However, the lattice parameters of the sol-gel products were slightly lower. As presented in the literature [
1,
2], there is an asymmetric solubility gap in the geikielite with dissolving some amount of Ti at elevated temperatures. The lattice parameters of sample GQ (MgTiO
3 with a small amount of Mg
2TiO
4) fit those of stoichiometric MgTiO3 as reported in Reference [
4]. This supports the phase diagram [
1,
2] that there is no Mg solubility. Since sample Kar2 [
7,
8] was a mixture of small amounts of MgTiO
3 and TiO
2 with MgTi
2O
5 as a major compound, it is reasonable to attribute the slight decrease of the lattice parameter of MgTiO
3 as stated in Reference [
8] to some excess of Ti. In order to verify this hypothesis, we conducted an additional HT-XRD study of a second sol-gel product with a mixture of MgTiO
3 and MgTi
2O
5. The xerogel with 1 < Mg:Ti < 2 was annealed 5 h at 1200 °C forming 78 wt% geikielite and 22 wt% karrooite. We designated this sample as “GK”.
Figure 5 shows that at elevated temperatures, the lattice parameters of the MgTiO
3 as obtained from HT-XRD were slightly higher than those in sample GQ and in reference [
4]. Moreover, they fit very well with those of sample Kar2 [
7,
8]. This confirms our assumption that the gap between the lattice parameters originated from excess of Ti in the Kar2 geikielite sample from references [
7,
8].
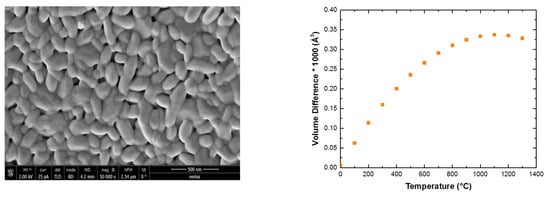
The difference between the lattice volumes for sample
GQ+ Reference [
4] and sample
GK+kar2 versus temperature is given in
Figure 6, which shows that the gap between the lattice parameters increased with temperature until 1000 °C and then slightly decreased.
In this work, stoichiometric geikielite from eight samples made by sol-gel technique yielded new HT-XRD data. Therefore, as a result of the present work, it was found that there is no single set of TECs for the geikielite. It agrees with the phase diagram determined by Shindo [
1] with an asymmetric solubility range in the geikielite. Both the sol-gel sample (GK) measured by HT-XRD and solid-state reaction (Kar2) measured by HT-ND were mixtures of geikielite and karrooite with maximum excess of Ti in the geikielite. Furthermore, both GK and Kar2 samples had similar lattice parameters, higher than the new data of the stoichiometric geikielite. Neither sample preparation nor diffraction method modified the TECs in geikielite. The fact that the sample GQ data integrated in seven stoichiometric samples [
4] agrees with the phase diagram determined by Shindo [
1] with absence of Mg solubility.