Iron-Doped Lithium Tantalate Thin Films Deposited by Magnetron Sputtering: A Study of the Iron Role in the Structure and the Derived Magnetic Properties
Abstract
:1. Introduction
2. Experimental Details
3. Results and Discussion
3.1. Structural and Morphological Properties of the LT NPs, T1, and T2
3.2. Structural and Morphological Properties of the LTO:Fe-LC and LTO:Fe-HC Thin Films
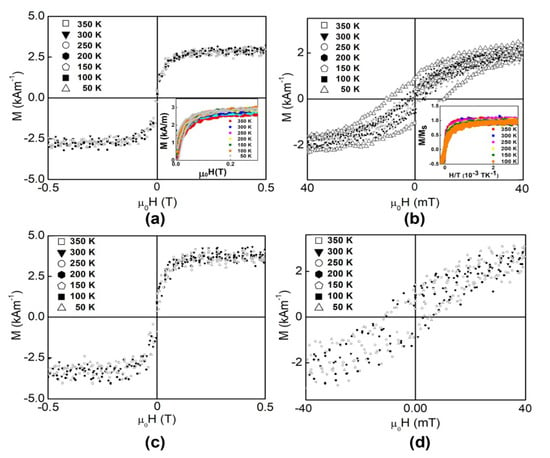
3.3. Magnetic Properties of the LTO:Fe-HC and LTO:Fe-LC Thin Films
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Ishihara, T. Inorganic Perovskite Oxides. In Springer Handbook of Electronic and Photonic Materials; Kasap, S., Peter, C., Eds.; Springer International Publishing: Cham, Switzerland, 2017; p. 1. [Google Scholar]
- Chen, C.; Zeng, F.; Li, J.H.; Sheng, P.; Luo, J.T.; Yang, Y.C.; Pan, F.; Zou, Y.; Huang, Y.Y.; Jiang, Z. Strong d–d electron interaction inducing ferromagnetism in Mn-doped LiNbO3. Thin Solid Films 2011, 520, 764–768. [Google Scholar] [CrossRef]
- Yao, D.; Zhou, X.; Ge, S. Raman scattering and room temperature ferromagnetism in Co-doped SrTiO3 particles. Appl. Surf. Sci. 2011, 257, 9233–9236. [Google Scholar] [CrossRef]
- Verma, K.C.; Gupta, V.; Kaur, J.; Kotnala, R.K. Raman spectra, photoluminescence, magnetism and magnetoelectric coupling in pure and Fe doped BaTiO3 nanostructures. J. Alloys Compd. 2013, 578, 5–11. [Google Scholar] [CrossRef]
- Song, Y.J.; Zhang, Q.H.; Shen, X.; Ni, X.D.; Yao, Y.; Yu, R.C. Room-Temperature Magnetism Realized by Doping Fe into Ferroelectric LiTaO3. Chin. Phys. Lett. 2014, 31, 017501. [Google Scholar] [CrossRef]
- Zeng, F.; Sheng, P.; Tang, G.S.; Pan, F.; Yan, W.S.; Hu, F.C.; Zou, Y.; Huang, Y.Y.; Jiang, Z.; Guo, D. Electronic structure and magnetism of Fe-doped LiNbO3. Mater. Chem. Phys. 2012, 136, 783–788. [Google Scholar] [CrossRef]
- Volk, T.; Wöhlecke, M. Lithium Niobate: Defects, Photorefraction and Ferroelectric Switching, 1st ed.; Hull, R., Osgood, R.M., Parisi, J., Warlimont, H., Eds.; Springer: Berlin, Germany, 2008; ISBN 3540707662. [Google Scholar]
- Takasugi, S.; Tomita, K.; Iwaoka, M.; Kato, H.; Kakihana, M. The hydrothermal and solvothermal synthesis of LiTaO3 photocatalyst: Suppressing the deterioration of the water splitting activity without using a cocatalyst. Int. J. Hydrog. Energy 2015, 40, 5638–5643. [Google Scholar] [CrossRef]
- Yang, T.; Liu, Y.; Zhang, L.; Hu, M.; Yang, Q.; Huang, Z.; Fang, M. Powder synthesis and properties of LiTaO3 ceramics. Adv. Powder Technol. 2014, 25, 933–936. [Google Scholar] [CrossRef]
- Gutmann, E.; Benke, A.; Gerth, K.; Böttcher, H.; Mehner, E.; Klein, C.; Krause-Buchholz, U.; Bergmann, U.; Pompe, W.; Meyer, D.C. Pyroelectrocatalytic Disinfection Using the Pyroelectric Effect of Nano- and Microcrystalline LiNbO3 and LiTaO3 Particles. J. Phys. Chem. C 2012, 116, 5383–5393. [Google Scholar] [CrossRef] [Green Version]
- Bamba, N.; Takaoka, J.; Chino, T.; Fukami, T.; Elouadi, B. Improved Piezoelectric Properties of LiTaO3 Family Solid Solution Ceramics with Modified Composition. Jpn. J. Appl. Phys. 2006, 45, 7426–7430. [Google Scholar] [CrossRef]
- Sun, B.; Wang, J.; Gou, J.; Liu, X.; Jiang, Y. Influence of thermal annealing on structural and optical properties of RF-sputtered LiTaO3 thin films. Mater. Res. Express 2018, 6, 026405. [Google Scholar] [CrossRef]
- Pan, D.; Wang, J.; Sun, B.; Gou, J. Study of the crystalline and optical properties of lithium tantalate thin films deposited by high power RF magnetron sputtering. Mater. Res. Express 2017, 4, 026402. [Google Scholar] [CrossRef]
- Combette, P.; Nougaret, L.; Giani, A.; Pascal-delannoy, F. RF magnetron-sputtering deposition of pyroelectric lithium tantalate thin films on ruthenium dioxide. J. Cryst. Growth 2007, 304, 90–96. [Google Scholar] [CrossRef]
- Nougaret, L.; Combette, P.; Pascal-Delannoy, F. Growth of lithium tantalate thin films by radio-frequency magnetron sputtering with lithium enriched target. Thin Solid Films 2009, 517, 1784–1789. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Qian, W.; Li, K.; Xie, J.S. Ferroelectric Property of Ion Beam Enhanced Deposited Lithium Tantalate Thin Film. Adv. Mater. Res. 2011, 335, 1418–1423. [Google Scholar] [CrossRef]
- Riefer, A.; Sanna, S.; Schmidt, W.G. LiNb1-xTaxO3 Electronic Structure and Optical Response from First-Principles Calculations. Ferroelectrics 2013, 447, 78–85. [Google Scholar] [CrossRef]
- He, X.; Li, K.; Liu, M.; He, Y.; Zhang, X.; Zhao, Y.; Xue, D. An optical spectroscopy study of defects in lithium tantalate single crystals. Opt. Commun. 2008, 281, 2531–2534. [Google Scholar] [CrossRef]
- Bhatt, R.; Bhaumik, I.; Ganesamoorthy, S.; Bright, R.; Soharab, M.; Karnal, A.; Gupta, P. Control of Intrinsic Defects in Lithium Niobate Single Crystal for Optoelectronic Applications. Crystals 2017, 7, 23. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.G.; Chiu, M. Substitution site of the Fe3+ impurity in crystalline LiNbO3. Phys. Rev. B 1994, 49, 12556–12558. [Google Scholar] [CrossRef]
- Gog, T.; Schotters, P.; Falta, J.; Materlik, G.; Grodzicki, M. The lattice position of Fe in Fe-doped LiNbO 3. J. Phys. Condens. Matter 1995, 7, 6971–6980. [Google Scholar] [CrossRef]
- Mignoni, S.; Fontana, M.D.; Bazzan, M.; Ciampolillo, M.V.; Zaltron, A.M.; Argiolas, N.; Sada, C. Micro-Raman analysis of Fe-diffused lithium niobate waveguides. Appl. Phys. B 2010, 101, 541–546. [Google Scholar] [CrossRef]
- Li, Y.Y.; Chen, H.L.; Chen, G.J.; Kuo, C.L.; Hsieh, P.H.; Hwang, W.S. Investigation of the Defect Structure of Congruent and Fe-Doped LiNbO3 Powders Synthesized by the Combustion Method. Materials 2017, 10, 380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitova, T.; Hormes, J.; Falk, M.; Buse, K. Site-selective investigation of site symmetry and site occupation of iron in Fe-doped lithium niobate crystals. J. Appl. Phys. 2009, 105, 013524. [Google Scholar] [CrossRef] [Green Version]
- Olimov, K.; Falk, M.; Buse, K.; Woike, T.; Hormes, J.; Modrow, H. X-ray absorption near edge spectroscopy investigations of valency and lattice occupation site of Fe in highly iron-doped lithium niobate crystals. J. Phys. Condens. Matter 2006, 18, 5135–5146. [Google Scholar] [CrossRef]
- Bush, T.S.; Catlow, C.R.A.; Chadwick, A.V.; Cole, M.; Geatches, R.M.; Greaves, G.N.; Tomlinson, S.M. Studies of cation dopant sites in metal oxides by EXAFS and computer-simulation techniques. J. Mater. Chem. 1992, 2, 309. [Google Scholar] [CrossRef]
- Alfonso, E.; Olaya, J.; Cubillos, G. Thin Film Growth Through Sputtering Technique and Its Applications. In Crystallization—Science and Technology; Barsi Andreeta, M.R., Ed.; InTech: Rijeka, Croatia, 2012; pp. 398–432. [Google Scholar]
- Crystallography Open Database. Available online: http://www.crystallography.net/cod/2101846.html (accessed on 3 September 2018).
- Sanna, S.; Neufeld, S.; Rüsing, M.; Berth, G.; Zrenner, A.; Schmidt, W.G. Raman scattering efficiency in LiTaO3 and LiNbO3 crystals. Phys. Rev. B 2015, 91, 224302. [Google Scholar] [CrossRef]
- Repelin, Y.; Husson, E.; Bennani, F.; Proust, C. Raman spectroscopy of lithium niobate and lithium tantalate. Force field calculations. J. Phys. Chem. Solids 1999, 60, 819–825. [Google Scholar] [CrossRef]
- Klug, H.P.; Harold, P.; Alexander, L.E.; Leroy, E. X-ray Diffraction Procedures for Polycrystalline and Amorphous Materials, 2nd ed.; John Wiley & Sons, Ltd.: New York, NY, USA, 1974; ISBN 9780471493693. [Google Scholar]
- Rahaman, M.N. Ceramic Processing and Sintering, 2nd ed.; Dekker, M., Ed.; M. Dekker: New York, NY, USA, 2003; ISBN 9780824709884. [Google Scholar]
- Shi, L.; Kong, Y.; Yan, W.; Sun, J.; Chen, S.; Zhang, L.; Zhang, W.; Liu, H.; Li, X.; Xie, X.; et al. Determination of the composition of lithium tantalate by means of Raman and OH− absorption measurements. Mater. Chem. Phys. 2006, 95, 229–234. [Google Scholar] [CrossRef]
- Fierro-Ruiz, C.; Sánchez-Dena, O.; Cabral-Larquier, E.; Elizalde-Galindo, J.; Farías, R. Structural and Magnetic Behavior of Oxidized and Reduced Fe Doped LiNbO3 Powders. Crystals 2018, 8, 108. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.Y.; Chen, H.L.; Kuo, C.L.; Hsieh, P.H.; Hwang, W.S. Raman spectra and ferromagnetism of nanocrystalline Fe-doped Li0.43Nb0.57O3+δ. Ceram. Int. 2016, 42, 10764–10769. [Google Scholar] [CrossRef]
- Fontana, M.D.; Bourson, P. Microstructure and defects probed by Raman spectroscopy in lithium niobate crystals and devices. Appl. Phys. Rev. 2015, 2, 40602. [Google Scholar] [CrossRef]
- Yadav, A.K.; Singh, P. A review of the structures of oxide glasses by Raman spectroscopy. RSC Adv. 2015, 5, 67583–67609. [Google Scholar] [CrossRef]
- Berengue, O.M.; Rodrigues, A.D.; Dalmaschio, C.J.; Lanfredi, A.J.C.; Leite, E.R.; Chiquito, A.J. Structural characterization of indium oxide nanostructures: A Raman analysis. J. Phys. D Appl. Phys. 2010, 43, 45401. [Google Scholar] [CrossRef]
- Bachiri, E.; El Bachiri, A.; El Hasnaoui, M.; Bennani, F.; Bousselamti, M. Effect of Ni-doping Charge on Structure and Properties of LiNbO3. J. Mater. Environ. Sci. 2016, 7, 3353–3361. [Google Scholar]
- Cullity, B.D.; Graham, C.D. Introduction to Magnetic Materials, 2nd ed.; Hanzo, L., Ed.; IEEE/Wiley: Hoboken, NJ, USA, 2009; ISBN 9780471477419. [Google Scholar]
- Chen, C.C.; Hsu, Y.J.; Lin, Y.F.; Lu, S.Y. Superparamagnetism Found in Diluted Magnetic Semiconductor Nanowires: Mn-Doped CdSe. J. Phys. Chem. C 2008, 112, 17964–17968. [Google Scholar] [CrossRef]
- Fonseca, F.C.; Goya, G.F.; Jardim, R.F.; Muccillo, R.; Carreño, N.L.V.; Longo, E.; Leite, E.R. Superparamagnetism and magnetic properties of Ni nanoparticles embedded in SiO2. Phys. Rev. B 2002, 66, 104406. [Google Scholar] [CrossRef] [Green Version]
- Borges, R.P.; da Silva, R.C.; Cruz, M.M.; Godinho, M. Influence of structural transitions of BaTiO3 on the magnetic properties of Fe nanoparticles. J. Phys. Conf. Ser. 2010, 200, 072014. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.; Yao, T.; Pan, Z.; Mai, C.; Sun, Z.; Wu, Z.; Hu, F.; Jiang, Y.; Yan, W. Role of Co clusters in wurtzite Co:ZnO dilute magnetic semiconductor thin films. J. Appl. Phys. 2009, 105, 043903. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, M.G.; Jang, H.M.; Ryu, S.; Kim, Y.M. Co-metal clustering as the origin of ferromagnetism in Co-doped ZnO thin films. Appl. Phys. Lett. 2004, 84, 1338–1340. [Google Scholar] [CrossRef] [Green Version]
- Shinde, S.R.; Ogale, S.B.; Higgins, J.S.; Zheng, H.; Millis, A.J.; Kulkarni, V.N.; Ramesh, R.; Greene, R.L.; Venkatesan, T. Co-occurrence of Superparamagnetism and Anomalous Hall Effect in Highly Reduced Cobalt-Doped Rutile TiO2−δ Films. Phys. Rev. Lett. 2004, 92, 166601. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Zuo, Z.; Zhou, H.M.; Beyermann, W.P.; Liu, J.L. Epitaxial Mn-doped ZnO diluted magnetic semiconductor thin films grown by plasma-assisted molecular-beam epitaxy. J. Cryst. Growth 2011, 314, 97–103. [Google Scholar] [CrossRef]
- Coey, J.M.D.; Mlack, J.T.; Venkatesan, M.; Stamenov, P. Magnetization Process in Dilute Magnetic Oxides. IEEE Trans. Magn. 2010, 46, 2501–2503. [Google Scholar] [CrossRef]
- Coey, J.M.D.; Venkatesan, M.; Fitzgerald, C.B. Donor impurity band exchange in dilute ferromagnetic oxides. Nat. Mater. 2005, 4, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Ackland, K.; Monzon, L.M.A.; Venkatesan, M.; Coey, J.M.D. Magnetism of Nanostructured CeO2. IEEE Trans. Magn. 2011, 47. [Google Scholar] [CrossRef]
- Mosivand, S.; Monzon, L.M.A.; Ackland, K.; Kazeminezhad, I.; Coey, J.M.D. Structural and magnetic properties of sonoelectrocrystallized magnetite nanoparticles. J. Phys. D Appl. Phys. 2014, 47, 13. [Google Scholar] [CrossRef]
- Coey, J.M.D. Dilute magnetic oxides. Curr. Opin. Solid State Mater. Sci. 2006, 10, 83–92. [Google Scholar] [CrossRef]
- Yadav, A.K.; Haque, S.M.; Shukla, D.; Choudhary, R.J.; Jha, S.N.; Bhattacharyya, D. X-ray absorption spectroscopy of Mn doped ZnO thin films prepared by rf sputtering technique. AIP Adv. 2015, 5. [Google Scholar] [CrossRef] [Green Version]
- Hu, L.; Zhu, L.; He, H.; Zhang, L.; Ye, Z. Acceptor defect-participating magnetic exchange in ZnO:Cu nanocrystalline film: Defect structure evolution, Cu-N synergetic role and magnetic control. J. Mater. Chem. C 2015, 3, 1330–1346. [Google Scholar] [CrossRef]
- Singhal, R.K.; Dhawan, M.S.; Gaur, S.K.; Dolia, S.N.; Kumar, S.; Shripathi, T.; Deshpande, U.P.; Xing, Y.T.; Saitovitch, E.; Garg, K.B. Study of ferromagnetism in Mn doped ZnO dilute semiconductor system. J. Phys. Conf. Ser. 2009, 153. [Google Scholar] [CrossRef]
- Rajkumar, N.; Susila, V.M.; Ramachandran, K. On the possibility of ferromagnetism in CdO:Mn at room temperature. J. Exp. Nanosci. 2011, 6, 389–398. [Google Scholar] [CrossRef]
- Depla, D.; Mahieu, S.; Greene, J.E. Sputter Deposition Processes. In Handbook of Deposition Technologies for Films and Coatings; Elsevier Inc.: Amsterdam, The Netherlands, 2010; pp. 253–296. ISBN 9780815520313. [Google Scholar]
- Khalaf, M.K.; Al-Taay, H.F.; Ali, D.S. Effect of radio frequency magnetron sputtering power on structural and optical properties of Ti6Al4V thin films. Photonic Sens. 2017, 7, 163–170. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.; Sun, Y.; Hing, P. The influence of deposition conditions on structure and morphology of aluminum nitride films deposited by radio frequency reactive sputtering. Thin Solid Films 2003, 434, 112–120. [Google Scholar] [CrossRef]
- Degen, T.; Sadki, M.; Bron, E.; König, U.; Nénert, G. The HighScore suite. Powder Diffr. 2014, 29, S13–S18. [Google Scholar] [CrossRef] [Green Version]
- Holland, T.J.B.; Redfern, S.A.T. Unit cell refinement from powder diffraction data: The use of regression diagnostics. Mineral. Mag. 1997, 61, 65–77. [Google Scholar] [CrossRef]









| LTO:Fe-LC | LTO:Fe-HC | |
|---|---|---|
| Target | T1 (LT-0.5%wt. Fe) | T2 (LT-1.8%wt. Fe) |
| Atmosphere | Argon | Argon |
| Handling pressure | 0.4 Pa | 0.4 Pa |
| R.F. gun power | 30 W | 50 W |
| Substrate temperature | 450 °C | 450 °C |
| Substrate-target distance | 16 cm | 16 cm |
| Deposition time | 1.5 h | 2.5 h |
| Lattice Parameters (Å) | Unit Cell Volume (Å3) | Weighted R Profile | Goodness of Fit | ||
|---|---|---|---|---|---|
| a = b | c | ||||
| LT powders | 5.155 | 13.766 | 316.79 | 8.21 | 4.08 |
| T1 | 5.154 | 13.780 | 316.99 | 9.26 | 5.03 |
| T2 | 5.152 | 13.783 | 316.82 | 10.38 | 2.05 |
| LTO:Fe-HC | 5.140 | 13.753 | 314.66 | 5.73 | 1.25 |
| LTO:Fe-LC | 5.149 | 13.700 | 314.59 | Sigmafit = 4.96 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villalobos Mendoza, S.D.; Holguín Momaca, J.T.; Elizalde Galindo, J.T.; Carrillo Flores, D.M.; Olive Méndez, S.F.; Farías Mancilla, J.R. Iron-Doped Lithium Tantalate Thin Films Deposited by Magnetron Sputtering: A Study of the Iron Role in the Structure and the Derived Magnetic Properties. Crystals 2020, 10, 50. https://doi.org/10.3390/cryst10010050
Villalobos Mendoza SD, Holguín Momaca JT, Elizalde Galindo JT, Carrillo Flores DM, Olive Méndez SF, Farías Mancilla JR. Iron-Doped Lithium Tantalate Thin Films Deposited by Magnetron Sputtering: A Study of the Iron Role in the Structure and the Derived Magnetic Properties. Crystals. 2020; 10(1):50. https://doi.org/10.3390/cryst10010050
Chicago/Turabian StyleVillalobos Mendoza, Sergio David, José Trinidad Holguín Momaca, José Trinidad Elizalde Galindo, Diana María Carrillo Flores, Sion Federico Olive Méndez, and José Rurik Farías Mancilla. 2020. "Iron-Doped Lithium Tantalate Thin Films Deposited by Magnetron Sputtering: A Study of the Iron Role in the Structure and the Derived Magnetic Properties" Crystals 10, no. 1: 50. https://doi.org/10.3390/cryst10010050