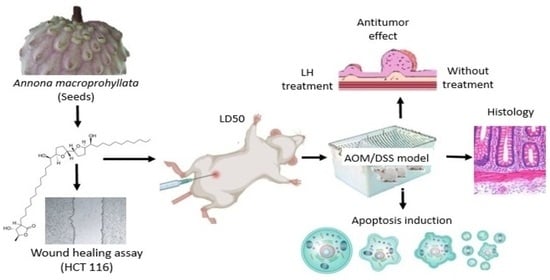
Laherradurin Inhibits Tumor Growth in an Azoxymethane/Dextran Sulfate Sodium Colorectal Cancer Model In Vivo
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Acute Toxicity (LD50)
2.2. AOM/DSS Model
2.2.1. Weight and Disease Activity Index (DAI)
2.2.2. Blood Analysis
2.2.3. Macroscopic Analysis of the Colon of Balb/c Mice
2.2.4. Histology Image Analysis
2.2.5. Apoptosis Induction by LH
2.2.6. Inhibition of Migration (Wound Healing Assay)
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Isolation and Identification of LH
4.3. Acute Toxicity Determination of the Mean Lethal Dose (LD50)
- LD50 = [M0 + M1]/2;
- M0 = highest dose without mortality;
- M1 = lowest dose with at least one deceased.
4.4. In Vivo Model of Colon Cancer
4.4.1. Animals
4.4.2. Induction of Colon Cancer by AOM/DSS
4.4.3. Blood Test and Colon Macroscopic Analysis
4.5. Histology and Immunohistochemical Analysis
4.5.1. Hematoxylin–Eosin Staining
4.5.2. Terminal Deoxynucleotide Transferase-Mediated Deoxy-UTP Nick End Labeling (TUNEL) Analysis
4.6. Wound Healing Assay
4.7. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Zhu, G.; Pei, L.; Xia, H.; Tang, Q.; Bi, F. Role of oncogenic KRAS in the prognosis, diagnosis and treatment of colorectal cancer. Mol. Cancer 2021, 20, 143. [Google Scholar] [CrossRef]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Modest, D.P.; Pant, S.; Sartore-Bianchi, A. Treatment sequencing in metastatic colorectal cancer. Eur. J. Cancer 2019, 109, 70–83. [Google Scholar] [CrossRef]
- Diasio, R.B.; Innocenti, F.; Offer, S.M. Pharmacogenomic-Guided Therapy in Colorectal Cancer. Clin. Pharmacol. Ther. 2021, 110, 616–625. [Google Scholar] [CrossRef]
- Latchman, J.; Guastella, A.; Tofthagen, C. 5-fluorouracil toxicity and dihydropyrimidine dehydrogenase enzyme: Implications for practice. Clinical J. Oncol. Nurs. 2014, 18, 581–585. [Google Scholar]
- Zhang, L.; Xing, X.; Meng, F.; Wang, Y.; Zhong, D. Oral fluoropyrimidine versus intravenous 5-fluorouracil for the treatment of advanced gastric and colorectal cancer: Meta-analysis. J. Gastroenterol. Hepatol. 2018, 33, 209–225. [Google Scholar] [CrossRef]
- Vodenkova, S.; Buchler, T.; Cervena, K.; Veskrnova, V.; Vodicka, P.; Vymetalkova, V. 5-fluorouracil and other fluoropyrimidines in colorectal cancer: Past, present and future. Pharmacol. Ther. 2020, 206, 107447. [Google Scholar] [CrossRef] [PubMed]
- Paulík, A.; Nekvindová, J.; Filip, S. Irinotecan toxicity during treatment of metastatic colorectal cancer: Focus on pharmacogenomics and personalized medicine. Tumori 2020, 106, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Karthika, C.; Sureshkumar, R.; Zehravi, M.; Akter, R.; Ali, F.; Ramproshad, S.; Mondal, B.; Kundu, M.K.; Dey, A.; Rahman, M.H.; et al. Multidrug Resistance in Cancer Cells: Focus on a Possible Strategy Plan to Address Colon Carcinoma Cells. Life 2022, 12, 811. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J. Natural products and drug discovery. Natl. Sci. Rev. 2022, 9, nwac206. [Google Scholar] [CrossRef]
- Alonso-Castro, A.J. Use of medicinal fauna in Mexican traditional medicine. J. Ethnopharmacol. 2014, 152, 53–70. [Google Scholar] [CrossRef]
- Jacobo-Herrera, N.J.; Jacobo-Herrera, F.E.; Zentella-Dehesa, A.; Andrade-Cetto, A.; Heinrich, M.; Pérez-Plasencia, C. Medicinal plants used in Mexican traditional medicine for the treatment of colorectal cancer. J. Ethnopharmacol. 2016, 179, 391–402. [Google Scholar] [CrossRef]
- Tormo, J.R.; Royo, I.; Gallardo, T.; Zafra-Polo, M.C.; Hernández, P.; Cortes, D.; Peláez, F. In vitro antitumor structure-activity relationships of threo/trans/threo mono-tetrahydrofuranic acetogenins: Correlations with their inhibition of mitochondrial complex I. Oncol. Res. 2003, 14, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.P.; Rachagani, S.; Purohit, V.; Pandey, P.; Joshi, S.; Moore, E.D.; Johansson, S.L.; Singh, P.K.; Ganti, A.K.; Batra, S.K. Graviola: A novel promising natural-derived drug that inhibits tumorigenicity and metastasis of pancreatic cancer cells in vitro and in vivo through altering cell metabolism. Cancer Lett. 2012, 323, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Moghadamtousi, S.Z.; Fadaeinasab, M.; Nikzad, S.; Mohan, G.; Ali, H.M.; Kadir, H.A. Annona muricata (Annonaceae): A Review of Its Traditional Uses, Isolated Acetogenins and Biological Activities. Int. J. Mol. Sci. 2015, 16, 15625–15658. [Google Scholar] [CrossRef] [PubMed]
- Savithramma, N.; Rao, M.L.; Suhrulatha, D. Screening of medicinal plants for secondary metabolites. MEJSR 2011, 8, 579–584. [Google Scholar]
- Durán, A.G.; Gutiérrez, M.T.; Mejías, F.J.R.; Molinillo, J.M.G.; Macías, F.A. An Overview of the Chemical Characteristics, Bioactivity and Achievements Regarding the Therapeutic Usage of Acetogenins from Annona cherimola Mill. Molecules 2021, 26, 2926. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Castro, A.J.; Villarreal, M.L.; Salazar-Olivo, L.A.; Gomez-Sanchez, M.; Dominguez, F.; Garcia-Carranca, A. Mexican medicinal plants used for cancer treatment: Pharmacological, phytochemical and ethnobotanical studies. J. Ethnopharmacol. 2011, 133, 945–972. [Google Scholar] [CrossRef] [PubMed]
- Brindis, F.; González-Trujano, M.E.; González-Andrade, M.; Aguirre-Hernández, E.; Villalobos-Molina, R. Aqueous extract of Annona macroprophyllata: A potential α-glucosidase inhibitor. Biomed. Res. Int. 2013, 2013, 591313. [Google Scholar] [CrossRef] [PubMed]
- Schlie-Guzmán, M.A.; García-Carrancá, A.; González-Esquinca, A.R. In vitro and in vivo antiproliferative activity of laherradurin and cherimolin-2 of Annona diversifolia Saff. Phytother. Res. 2009, 23, 1128–1133. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Ma, Q.; Ye, L.; Piao, G. The Traditional Medicine and Modern Medicine from Natural Products. Molecules 2016, 21, 559. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Sen, T.; Samanta, S.K. Medicinal plants, human health and biodiversity: A broad review. Adv. Biochem. Eng. Biotechnol. 2015, 147, 59–110. [Google Scholar]
- World Health Organization. WHO Global Centre for Traditional Medicine. Available online: https://www.who.int/initiatives/who-global-centre-for-traditional-medicine (accessed on 6 November 2023).
- Tormo, J.R.; De Pedro, N.; Royo, I.; Barrachina, I.; Zafra-Polo, M.C.; Cuadrillero, C.; Hernández, P.; Cortes, D.; Peláez, F. In vitro antitumor structure-activity relationships of threo/trans/threo/trans/erythro bis-tetrahydrofuranic acetogenins: Correlations with their inhibition of mitochondrial complex I. Oncol. Res. 2005, 15, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Tantithanaporn, S.; Wattanapiromsakul, C.; Itharat, A.; Keawpradub, N. Cytotoxic activity of acetogenins and styryl lactones isolated from Goniothalamus undulatus Ridl. root extracts against a lung cancer cell line (COR-L23). Phytomedicine 2011, 18, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Yuan, F.; Bai, G.; Chen, Y.; Miao, Y.; Chen, J.; Li, X. Structure-activity relationships of diverse ACGs against multidrug resistant human lung cancer cell line A549/Taxol. Bioorg Med. Chem. Lett. 2015, 25, 787–790. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.Q.; Sun, P.; Pan, Z.Y.; Fang, Z.Z. Annonaceous acetogenins reverse drug resistance of human hepatocellular carcinoma BEL-7402/5-FU and HepG2/ADM cell lines. Int. J. Clin. Exp. Pathol. 2015, 8, 11934–11944. [Google Scholar]
- Chen, Y.; Chen, J.W.; Wang, Y.; Xu, S.S.; Li, X. Six cytotoxic annonaceous acetogenins from Annona squamosa seeds. Food Chem. 2012, 135, 960–966. [Google Scholar] [CrossRef]
- Dai, Y.; Hogan, S.; Schmelz, E.M.; Ju, Y.H.; Canning, C.; Zhou, K. Selective growth inhibition of human breast cancer cells by graviola fruit extract in vitro and in vivo involving downregulation of EGFR expression. Nutr. Cancer 2011, 63, 795–801. [Google Scholar] [CrossRef]
- Griggs, N.D.; Phillips, A.J. A concise and modular synthesis of pyranicin. Org. Lett. 2008, 10, 4955–4957. [Google Scholar] [CrossRef]
- Ko, Y.M.; Wu, T.Y.; Wu, Y.C.; Chang, F.R.; Guh, J.Y.; Chuang, L.Y. Annonacin induces cell cycle-dependent growth arrest and apoptosis in estrogen receptor-α-related pathways in MCF-7 cells. J. Ethnopharmacol. 2011, 37, 1283–1290. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, J.; Xu, S.; Wang, Y.; Li, X.; Cai, B. Antitumor activity of annonaceous acetogenins in HepS and S180 xenografts bearing mice. Bioorg. Med. Chem. Lett. 2012, 22, 2717–2719. [Google Scholar] [CrossRef]
- Yang, C.; Gundala, S.R.; Mukkavilli, R.; Vangala, S.; Reid, M.D.; Aneja, R. Synergistic interactions among flavonoids and acetogenins in Graviola (Annona muricata) leaves confer protection against prostate cancer. Carcinogenesis 2015, 36, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Livshits, Z.; Rao, R.B.; Smith, S.W. An approach to chemotherapy-associated toxicity. Emerg. Med. Clin. N. Am. 2014, 32, 167–203. [Google Scholar] [CrossRef] [PubMed]
- Simkens, L.H.J.; Koopman, M.; Punt, C.J.A. Optimal duration of systemic treatment in metastatic colorectal cancer. Curr. Opin. Oncol. 2014, 26, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.W.; Saif, M.W.; El-Rayes, B.F.; Fakih, M.G.; Cartwright, T.H.; Posey, J.A.; King, T.R.; Von Borstel, R.W.; Bamat, M.K. Emergency use of uridine triacetate for the prevention and treatment of life-threatening 5-fluorouracil and capecitabine toxicity. Cancer 2017, 123, 345–356. [Google Scholar] [PubMed]
- OECD iLibrary. OECD Guidelines for the Testing of Chemicals, Section 4. Available online: https://www.oecd-ilibrary.org/environment/test-no-423-acute-oral-toxicity-acute-toxic-class-method_9789264071001-en (accessed on 12 October 2023).
- Durán-Ruiz, C.A.; Cruz-Ortega, R.; Zaldívar-Riverón, A.; Zavaleta-Mancera, H.A.; De-la-Cruz-Chacón, I.; González-Esquinca, A.R. Ontogenic synchronization of Bephratelloides cubensis, Annona macroprophyllata seeds and acetogenins from Annonaceae. J. Plant Res. 2019, 132, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Chen, Y.; Chen, J.; Li, X.; Chen, Y.A. Review on Annona squamosa L.: Phytochemicals and Biological Activities. Am. J. Chin. Med. 2017, 45, 933–964. [Google Scholar] [CrossRef]
- Mutakin, M.; Fauziati, R.; Fadhilah, F.N.; Zuhrotun, A.; Amalia, R.; Hadisaputri, Y.E. Pharmacological Activities of Soursop (Annona muricata Lin.). Molecules 2022, 27, 1201. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yeung, S.J.; Liu, S.; Qdaisat, A.; Jiang, D.; Liu, W.; Cheng, Z.; Liu, W.; Wang, H.; Li, L.; et al. Cyst(e)ine in nutrition formulation promotes colon cancer growth and chemoresistance by activating mTORC1 and scavenging ROS. Signal Transduct. Target. Ther. 2021, 6, 188. [Google Scholar] [CrossRef] [PubMed]
- Tisdale, M.J. Catabolic mediators of cancer cachexia. Curr. Opin. Support. Palliat. Care 2008, 2, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.K.; Acharyya, S. Understanding cachexia in the context of metastatic progression. Nat. Rev. Cancer 2020, 20, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.R.; Laird, B.J.A.; Wigmore, S.J.; Skipworth, R.J.E. Understanding Cancer Cachexia and Its Implications in Upper Gastrointestinal Cancers. Curr. Treat. Options Oncol. 2022, 23, 1732–1747. [Google Scholar] [CrossRef] [PubMed]
- Cid-Gallegos, M.S.; Jiménez-Martínez, C.; Sánchez-Chino, X.M.; Madrigal-Bujaidar, E.; Vásquez-Garzón, V.R.; Baltiérrez-Hoyos, R.; Álvarez-González, I. Chemopreventive Effect of Cooked Chickpea on Colon Carcinogenesis Evolution in AOM/DSS-Induced Balb/c Mice. Plants 2023, 12, 2317. [Google Scholar] [CrossRef]
- Sun, W.; Gao, J.; Yang, B.; Chen, X.; Kang, N.; Liu, W. Protocol for colitis-associated colorectal cancer murine model induced by AOM and DSS. STAR Protoc. 2023, 4, 102105. [Google Scholar] [CrossRef]
- Jialing, L.; Yangyang, G.; Jing, Z.; Xiaoyi, T.; Ping, W.; Liwei, S.; Simin, C. Changes in serum inflammatory cytokine levels and intestinal flora in a self-healing dextran sodium sulfate-induced ulcerative colitis murine model. Life Sci. 2020, 263, 118587. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, C.; Guo, C.; Li, X. Chitosan Ameliorates DSS-Induced Ulcerative Colitis Mice by Enhancing Intestinal Barrier Function and Improving Microflora. Int. J. Mol. Sci. 2019, 20, 5751. [Google Scholar] [CrossRef]
- Eichele, D.D.; Kharbanda, K.K. Dextran sodium sulfate colitis murine model: An indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J. Gastroenterol. 2017, 33, 6016–6029. [Google Scholar] [CrossRef]
- Modesto, R.; Estarreja, J.; Silva, I.; Rocha, J.; Pinto, R.; Mateus, V. Chemically Induced Colitis-Associated Cancer Models in Rodents for Pharmacological Modulation: A Systematic Review. J. Clin. Med. 2022, 11, 2739. [Google Scholar] [CrossRef]
- Weiss, G.; Goodnough, L.T. Anemia of chronic disease. N. Engl. J. Med. 2005, 352, 1011–1023. [Google Scholar] [CrossRef]
- Huang, X.Z.; Yang, Y.C.; Chen, Y.; Wu, C.C.; Lin, R.F.; Wang, Z.N.; Zhang, X. Preoperative Anemia or Low Hemoglobin Predicts Poor Prognosis in Gastric Cancer Patients: A Meta-Analysis. Dis. Markers 2019, 2019, 7606128. [Google Scholar] [CrossRef]
- Wilson, M.J.; van Haaren, M.; Harlaar, J.J.; Park, H.C.; Bonjer, H.J.; Jeekel, J.; Zwaginga, J.J.; Schipperus, M. Long-term prognostic value of preoperative anemia in patients with colorectal cancer: A systematic review and meta-analysis. Surg. Oncol. 2017, 26, 96–104. [Google Scholar] [CrossRef]
- Ju, J.; Lee, G.Y.; Kim, Y.S.; Chang, H.K.; Do, M.S.; Park, K.Y. Bamboo salt suppresses colon carcinogenesis in C57BL/6 mice with chemically induced colitis. J. Med. Food 2016, 19, 1015–1022. [Google Scholar] [CrossRef]
- Kim, H.Y.; Seo, J.E.; Lee, H.; Bae, C.H.; Ha, K.T.; Kim, S. Rumex japonicus Houtt. Extract Suppresses Colitis-Associated Colorectal Cancer by Regulating Inflammation and Tight-Junction Integrity in Mice. Front. Pharmacol. 2022, 5, 946909. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.C.; Suh, J.H.; Wang, Y.; Pahwa, M.; Badmaev, V.; Ho, C.T.; Pan, M.H. Boswellia serrata resin extract alleviates azoxymethane (AOM)/dextran sodium sulfate (DSS)-induced colon tumorigenesis. Mol. Nutr. Food Res. 2017, 61, 1600984. [Google Scholar] [CrossRef] [PubMed]
- Iskander, A.; Yan, L.J. Cisplatin-Induced Kidney Toxicity: Potential Roles of Major NAD+-Dependent Enzymes and Plant-Derived Natural Products. Biomolecules 2022, 12, 1078. [Google Scholar] [CrossRef] [PubMed]
- Yaegashi, A.; Yoshida, K.; Suzuki, N.; Shimada, I.; Tani, Y.; Saijo, Y.; Toyama, A. A case of severe hepatotoxicity induced by cisplatin and 5-fluorouracil. Int. Cancer Conf. J. 2019, 9, 24–27. [Google Scholar] [CrossRef]
- Albers, J.W.; Chaudhry, V.; Cavaletti, G.; Donehower, R.C. Interventions for preventing neuropathy caused by cisplatin and related com-pounds. Cochrane Database Syst. Rev. 2014, 3, 1–79. [Google Scholar]
- Callejo, A.; Sedó-Cabezón, L.; Juan, I.D.; Llorens, J. Cisplatin-Induced Ototoxicity: Effects, Mechanisms and Protection Strategies. Toxics 2015, 3, 268–293. [Google Scholar] [CrossRef]
- Rahimi, A.; Asadi, F.; Rezghi, M.; Kazemi, S.; Soorani, F.; Memariani, Z. Natural products against cisplatin-induced male reproductive toxicity: A comprehensive review. J. Biochem. Mol. Toxicol. 2022, 36, e22970. [Google Scholar] [CrossRef]
- Champy, P.; Höglinger, G.U.; Féger, J.; Gleye, C.; Hocquemiller, R.; Laurens, A.; Guérineau, V.; Laprévote, O.; Medja, F.; Lombès, A.; et al. Annonacin, a lipophilic inhibitor of mitochondrial complex I, induces nigral and striatal neurodegeneration in rats: Possible relevance for atypical parkinsonism in Guadeloupe. J. Neurochem. 2004, 88, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Champy, P.; Melot, A.; Guérineau Eng, V.; Gleye, C.; Fall, D.; Höglinger, G.U.; Ruberg, M.; Lannuzel, A.; Laprévote, O.; Laurens, A.; et al. Quantification of acetogenins in Annona muricata linked to atypical parkinsonism in guadeloupe. Mov. Disord. 2005, 20, 1629–1633. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Khondiker, M.; Höllerhage, M.; Muriel, M.P.; Champy, P.; Bach, A.; Depienne, C.; Respondek, G.; Yamada, E.S.; Lannuzel, A.; Yagi, T.; et al. Annonacin, a natural mitochondrial complex I inhibitor, causes tau pathology in cultured neurons. J. Neurosci. 2007, 27, 7827–7837. [Google Scholar] [CrossRef]
- Le Ven, J.; Schmitz-Afonso, I.; Lewin, G.; Brunelle, A.; Touboul, D.; Champy, P. Identification of the environmental neurotoxins annonaceous acetogenins in an Annona cherimolia Mill. Alcoholic Beverage Using HPLC-ESI-LTQ-Orbitrap. J. Agric. Food Chem. 2014, 62, 8696–8704. [Google Scholar] [CrossRef] [PubMed]
- Degli Esposti, M.; Ghelli, A.; Ratta, M.; Cortes, D.; Estornell, E. Natural substances (acetogenins) from the family Annonaceae are powerful inhibitors of mitochondrial NADH dehydrogenase (Complex I). Biochem. J. 1994, 301, 161–167. [Google Scholar] [CrossRef]
- Chang, F.R.; Wu, Y.C. Novel cytotoxic annonaceous acetogenins from Annona muricata. J. Nat. Prod. 2001, 64, 925–931. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Wang, T.D.; Shen, S.M.; Yu, Y.; Mao, C.; Yao, Z.J.; Wang, L.S. Annonaceous acetogenin mimic AA005 induces cancer cell death via apoptosis inducing factor through a caspase-3-independent mechanism. BMC Cancer 2015, 15, 139. [Google Scholar] [CrossRef] [PubMed]
- Gaviria-Calle, M.M.; Posada-Arias, S.; Mira-Hernández, J. Acetogeninas, alternativa en el tratamiento de cáncer en caninos. Rev. CES Med. Zootec. 2018, 13, 157–172. [Google Scholar] [CrossRef]
- Mangal, M.; Mhod, I.K.; Subhash, M.A. Acetogenins as Potential Anticancer Agents. Anticancer. Agents Med. Chem. 2016, 16, 138–159. [Google Scholar] [CrossRef]
- Yap, C.V.; Subramaniam, K.S.; Khor, S.W.; Chung, I. Annonacin Exerts Antitumor Activity through Induction of Apoptosis and Extracellular Signal-regulated Kinase Inhibition. Pharmacogn. Res. 2017, 9, 378–383. [Google Scholar]
- Yuan, S.S.; Chang, H.L.; Chen, H.W.; Kuo, F.C.; Liaw, C.C.; Su, J.H.; Wu, Y.C. Selective cytotoxicity of squamocin on T24 bladder cancer cells at the S-phase via a Bax-, Bad-, and caspase-3-related pathways. Life Sci. 2006, 78, 869–874. [Google Scholar] [CrossRef]
- Jacobo-Herrera, N.; Pérez-Plasencia, C.; Castro-Torres, V.A.; Martínez-Vázquez, M.; González-Esquinca, A.R.; Zentella-Dehesa, A. Selective Acetogenins and Their Potential as Anticancer Agents. Front. Pharmacol. 2019, 10, 783. [Google Scholar] [CrossRef] [PubMed]
- De Pedro, N.; Cautain, B.; Melguizo, A.; Vicente, F.; Genilloud, O.; Peláez, F.; Tormo, J.R. Mitochondrial complex I inhibitors, acetogenins, induce HepG2 cell death through the induction of the complete apoptotic mitochondrial pathway. J. Bioenerg. Biomembr. 2013, 45, 153–164. [Google Scholar] [CrossRef]
- Tsilimigras, D.I.; Ntanasis-Stathopoulos, I.; Pawlik, T.M. Molecular Mechanisms of Colorectal Liver Metastases. Cells 2023, 12, 1657. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Lee, N.H.; Son, C.G. A Review of Herbal Resources Inducing Anti-Liver Metastasis Effects in Gastrointestinal Tumors via Modulation of Tumor Microenvironments in Animal Models. Cancers 2023, 15, 3415. [Google Scholar] [CrossRef] [PubMed]
- Chinedu, E.; Arome, D.; Ameh, F.S. A new method for determining acute toxicity in animal models. Toxicol. Int. 2013, 20, 224–226. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar]
- Cuellar-Nuñez, M.L.; Luzardo-Ocampo, I.; Campos-Vega, R.; Gallegos-Corona, M.A.; González de Mejía, E.; Loarca-Piña, G. Physicochemical and nutraceutical properties of moringa (Moringa oleifera) leaves and their effects in an in vivo AOM/DSS-induced colorectal carcinogenesis model. Food Res. Int. 2018, 105, 159–168. [Google Scholar] [CrossRef]
- Boivin, G.P.; Washington, K.; Yang, K.; Ward, J.M.; Pretlow, T.P.; Russell, R.; Besselsen, D.G.; Godfrey, V.L.; Doetschman, T.; Dove, W.F.; et al. Pathology of mouse models of intestinal cancer: Consensus report and recommendations. Gastroenterology 2003, 124, 762–777. [Google Scholar] [CrossRef]
- Grada, A.; Otero-Vinas, M.; Prieto-Castrillo, F.; Obagi, Z.; Falanga, V. Research Techniques Made Simple: Analysis of Collective Cell Migration Using the Wound Healing Assay. J. Investig. Dermatol. 2017, 137, e11–e16. [Google Scholar] [CrossRef] [PubMed]








| Biochemical Analysis | NC | PC | Cisplatin (2.0 mg/Kg) | LH | |||
|---|---|---|---|---|---|---|---|
| 0.5 mg/Kg | 1.5 mg/Kg | 3.0 mg/Kg | 3.0 mg/Kg (Double) | ||||
| Erythrocytes (×1012/L) | 9.59 ± 0.34 | 5.42 ± 0.066 * | 5.99 ± 1.65 * | 5.29 ± 1.87 * | 8.64 ± 1.22 | 8.86 ± 0.78 | 8.99 ± 0.18 |
| Leukocytes (×109/L) | 7.75 ± 0.77 | 3.93 ± 0.15 * | 3.45 ± 0.77 * | 4.2 ± 2.82 * | 4 ± 0.7 * | 4.26 ± 0.141 * | 3.83 ± 0.30 * |
| Hematocrit (%) | 51.1 ± 1.2 | 27.2 ± 1.6 * | 31.2 ± 8.45 * | 34 ± 10.3 * | 38.66 ±6.57 | 43.92 ± 3.36 | 45.6 ± 1.21 |
| Lymphocytes (%) | 61.33 ± 6.11 | 85.33 ± 5.5 * | 74.66 ± 3.05 * | 86.5 ± 2.12 * | 86 ± 8.12 * | 82.175 ± 0.82 * | 85.66 ± 1.52 * |
| Neutrophils (%) | 34.66 ± 3.5 | 10.66 ± 7.23 * | 23.33 ± 1.15 * | 19.33 ± 10.21 * | 16 ± 8.5 * | 15 ± 1.15 * | 13.33 ± 0.57 * |
| Hemoglobin (g/L) | 157 ± 5.2 | 87.6 ± 4.1 * | 98.6 ± 21.7 * | 93.6 ± 23 * | 120.3 ± 18.4 | 136 ± 10.7 | 144 ± 9.5 |
| Glucose (g/L) | 1.19 ± 0.09 | 2.07 ± 0.14 * | 0.94 ± 0.09 | 1.65 ± 0.07 * | 1.19 ± 0.09 | 1.01 ± 0.04 | 1.08 ± 0.1 |
| Urea (g/L) | 0.44 ± 0.02 | 0.58 ± 0.15 * | 0.4 ± 0.06 | 0.4 ± 0.04 | 0.41 ± 0.03 | 0.36 ± 0.02 | 0.35 ± 0.01 |
| Creatinine (mg/dL) | 0.0056 ± 0.00049 | 0.0064 ± 0.00041 | 0.0055 ± 0.0007 | 0.006 ± 0.001 | 0.005 ± 0.00011 | 0.0057 ± 0.0012 | 0.0055 ± 0.0007 |
| Alanine transferase U/I | 132.7 ± 0.56 | 50.86 ± 11 * | 89.5 ± 3.5 * | 63 ± 2.8 * | 110.45 ± 1.76 * | 132 ± 3.6 | 126.33 ± 5.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rendón-Barrón, M.J.; Pérez-Arteaga, E.; Delgado-Waldo, I.; Coronel-Hernández, J.; Pérez-Plasencia, C.; Rodríguez-Izquierdo, F.; Linares, R.; González-Esquinca, A.R.; Álvarez-González, I.; Madrigal-Bujaidar, E.; et al. Laherradurin Inhibits Tumor Growth in an Azoxymethane/Dextran Sulfate Sodium Colorectal Cancer Model In Vivo. Cancers 2024, 16, 573. https://doi.org/10.3390/cancers16030573
Rendón-Barrón MJ, Pérez-Arteaga E, Delgado-Waldo I, Coronel-Hernández J, Pérez-Plasencia C, Rodríguez-Izquierdo F, Linares R, González-Esquinca AR, Álvarez-González I, Madrigal-Bujaidar E, et al. Laherradurin Inhibits Tumor Growth in an Azoxymethane/Dextran Sulfate Sodium Colorectal Cancer Model In Vivo. Cancers. 2024; 16(3):573. https://doi.org/10.3390/cancers16030573
Chicago/Turabian StyleRendón-Barrón, Michael Joshue, Eduardo Pérez-Arteaga, Izamary Delgado-Waldo, Jossimar Coronel-Hernández, Carlos Pérez-Plasencia, Frida Rodríguez-Izquierdo, Rosa Linares, Alma Rosa González-Esquinca, Isela Álvarez-González, Eduardo Madrigal-Bujaidar, and et al. 2024. "Laherradurin Inhibits Tumor Growth in an Azoxymethane/Dextran Sulfate Sodium Colorectal Cancer Model In Vivo" Cancers 16, no. 3: 573. https://doi.org/10.3390/cancers16030573