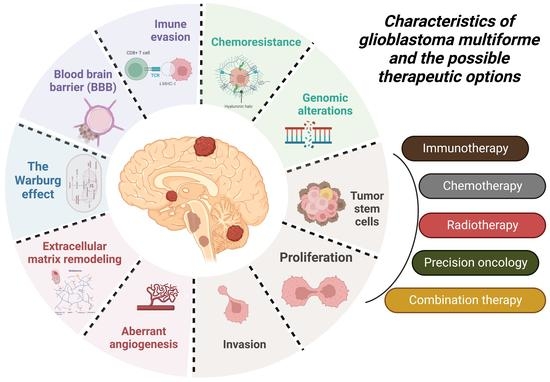
Mechanisms of Resistance and Current Treatment Options for Glioblastoma Multiforme (GBM)
Abstract
:Simple Summary
Abstract
1. Introduction
2. Conventional Treatment Options
2.1. Traditional Therapy
2.1.1. Surgical Resection
2.1.2. Cytotoxic and Anti-Angiogenic Chemotherapy
2.1.3. Radiation Therapy (RT)
2.2. Mechanisms of Resistance
2.2.1. DNA-Repair Enzymes
2.2.2. Mismatch-Repair (MMR) Complex Formation
2.2.3. Glioma Initiating Cells (GICs)
2.2.4. Hypoxia and Autophagy
3. Treatment Challenges
3.1. Blood–Brain Barrier (BBB)
3.2. Blood–Brain Tumor Barrier (BBTB)
3.3. Intra-Brain Tissue Diffusion
3.4. Chemo-Radiation Resistance of Cancer Stem Cells (CSCs) in GBM
3.5. Intertumoral Heterogeneity
3.6. Intratumoral Heterogeneity
3.7. Heterogeneity Caused by Extracellular Vesicles (EVs)
4. New Therapies for the Treatment of GBM
4.1. Immunotherapy
4.1.1. Non-Specific Passive Immunotherapy
- Direct cytotoxic effect (TNF-α);
- Modification of lymphocyte migration (TNF, IL-1, INF-γ) [112];
- Increased sensitivity of cancer cells to the cytotoxic effects of various biological or chemical agents (INF-γ, TNF-α) [113];
- Inhibition of tumor cell proliferation (INF-α, INF-γ);
- Activation of NK cells (GM-CSF, IL-2, IL-6) [112].
4.1.2. Specific Passive Immunotherapy
4.1.3. Non-Specific Active Immunotherapy
4.1.4. Specific Active Immunotherapy
- Non-cellular vaccines—peptide, HSP (heat shock protein—vaccines based on heat shock proteins), DNA, and viral vaccines;
- Cellular vaccines—unmodified and genetically modified, and DC cells “fed” with tumor antigens.
4.2. Inhibitors
4.3. Ketogenic Diet
| NCT Number | Title | Conditions | Interventions | Outcome Measures | Phases | Last Update Posted |
|---|---|---|---|---|---|---|
| NCT01865162 | Ketogenic Diet as Adjunctive Treatment in Refractory/End-stage Glioblastoma Multiforme: a Pilot Study | Glioblastoma Multiforme | ketogenic diet | To evaluate the safety of ketogenic diet as adjunctive treatment of treatment-refractory glioblastoma multiforme|To obtain pilot data on efficacy of ketogenic diet as adjunctive treatment of treatment-refractory glioblastoma multiforme|To evaluate tolerability of ketogenic diet as adjunctive treatment of treatment-refractory glioblastoma multiforme | Phase 1 | 9 November 2022 |
| NCT02302235 | Ketogenic Diet Treatment Adjunctive to Radiation and Chemotherapy in Glioblastoma Multiforme: a Pilot Study | Glioblastoma Multiforme of Brain | Ketogenic Diet Standardized Diet | Survival time|time to radiological (MRI) tumor progression|The incidence of treatment-emergent adverse events during treatment|Tolerability of ketogenic diet: rate of early discontinuation of subjects from the diet because of intolerability, defined as unwillingness by the subject to continue with the diet because of possible diet related side effects | Phase 2 | 11 August 2022 |
| NCT02939378 | Ketogenic Diet Adjunctive to Salvage Chemotherapy for Recurrent Glioblastoma: a Pilot Study | Glioblastoma Multiforme | Ketogenic diet Standard diet | Number of Participants with Treatment-emergent Adverse Effects|The Chemosensitivity of Tumor|Overall Survival|Ketosis|Quality of Life | Phase 1 Phase 2 | 20 October 2016 |
| NCT05708352 | A Phase 2 Study of the Ketogenic Diet vs. Standard Diet Guidance for Patients With Glioblastoma in Combination With Standard-of-care Treatment | Glioblastoma Multiforme | Behavioral: Keto Diet Behavioral: Usual Diet | Overall survival|Health-related quality of life 1|Health-related quality of life 2|Progression-free survival|Cognitive performance 1|Cognitive performance 2|Physical activity | Phase 2 | 2 February 2023 |
| NCT04691960 | A Pilot Study of Ketogenic Diet and Metformin in Glioblastoma: Feasibility and Metabolic Imaging | Glioblastoma | Ketogenic Diet Drug: Metformin | Ability to achieve and maintain ketosis|Tolerability of metformin | Phase 2 | 13 December 2022 |
| NCT00575146 | Ketogenic Diet for Recurrent Glioblastoma | Recurrent Glioblastoma | Dietary Supplement: TAVARLIN | Applicability as Measured by Discontinuation of Study Treatment Due to Intolerability|Progression-free survival|Overall Survival|Frequency of Seizures|Ketosis|Quality of Life | Phase 1 | 2 May 2014 |
| NCT03451799 | Ketogenic Diet in Combination With Standard-of-care Radiation and Temozolomide for Patients With Glioblastoma | GBM Glioblastoma | Ketogenic Diet Radiation: Standard-of-care radiation Drug: Standard-of-care Temozolomide | Safety of the intervention|Feasibility of the intervention|Overall Survival|Time-to-progression|Quality of Life (two months)|Quality of Life (four months)|Cognitive function (Hopkins Verbal Learning Test-Revised)|Cognitive function (Trail Making Test)|Cognitive function (Controlled Word Association Test)|Cognitive function (Montreal Cognitive Assessment) | Phase 1 | 13 January 2023 |
| NCT03075514 | Ketogenic Diets as an Adjuvant Therapy in Glioblastoma | Glioblastoma Glioblastoma Multiforme, Adult | MKD MCT | To assess retention and drop-out rates|Estimation of recruitment rates|Enrolment of patients Long-term retention|Dietary adjustments required to achieve ketosis|Self-reported dietary compliance|Calculated dietary compliance|MCT compliance|Ketosis levels|Dietetic time required for interventions|Protocol refinements required|Sample size estimates for future trials|Quality of life|Food acceptability|Gastrointestinal side effects|Changes to biochemical markers|Anthropometric changes|Completeness of data | Not Applicable | 4 April 2019 |
| NCT01754350 | Calorie-restricted, Ketogenic Diet and Transient Fasting During Reirradiation for Patients With Recurrent Glioblastoma | Recurrent Glioblastoma | Calorie-restricted ketogenic diet and transient fasting standard nutrition | Progression-free-survival|Feasibility Measured as Median Number of Days on Diet Per Patient and Average Calorie and Carbohydrate Intake Per Day During Day 1–9|Safety and Tolerability as Defined as Number of Patients With Adverse Events|Overall Survival|Frequency of Seizures|Ketosis|Quality of Life as Measured by the EORTC Quality of Life Questionnaire|Depression|Attention|Response | Not Applicable | 2 June 2021 |
| NCT02046187 | Ketogenic Diet With Radiation and Chemotherapy for Newly Diagnosed Glioblastoma | Glioblastoma (GBM) | Ketogenic Diet Radiation therapy Drug: Temozolomide | Number of participants with adverse events|overall survival|time to progression|quality of life | Phase 1 Phase 2 | 16 June 2021 |
| NCT05183204 | Paxalisib With a High Fat, Low Carb Diet and Metformin for Glioblastoma | Glioblastoma | Drug: Paxalisib Drug: Metformin Ketogenic Diet | Progression-free survival, defined as the survival rate at 6 months|Overall survival, defined as the time of first study treatment to death from any cause|Change in insulin levels|Change in tumor glucose uptake values | Phase 2 | 4 October 2022 |
| NCT04730869 | Metabolic Therapy Program In Conjunction With Standard Treatment For Glioblastoma Multiforme | Glioblastoma Multiforme | Standard Treatment Plus Metabolic Therapy Program | Mean daily blood glucose-to-ketone ratio during chemoradiation|Mean daily blood glucose-to-ketone ratio during adjuvant chemotherapy|Mean daily blood glucose-to-ketone ratio during the MTP, calculated separately on fasting and ketogenic diet days|Change in weight|Safety as measured by National Cancer Institute Common Terminology Criteria for Adverse Events (version 4)|Change in performance status as measured by Eastern Cooperative Oncology Group Performance Status scale|Change in leisure/exercise activity as measured by Godin Leisure-Time Exercise questionnaire|Change in quality of life as measured by Functional Assessment of Cancer Therapy—Brain questionnaire|Progression-free survival|Overall survival | Not Applicable | 2 June 2021 |
| NCT03278249 | Feasibility Study of Modified Atkins Ketogenic Diet in the Treatment of Newly Diagnosed Malignant Glioma | Glioblastoma | Modified Atkins Ketogenic Diet | Assessment of inducing ketosis|Assessment of progression-free survival|Assessment of survival | Not Applicable | 24 November 2021 |
| NCT01535911 | Pilot Study of a Metabolic Nutritional Therapy for the Management of Primary Brain Tumors | Glioblastoma | Energy restricted Ketogenic Diet (ERKD) (Metabolic Nutritional Therapy) | MRI imaging will be used to measure changes in brain tumor size. | Not Applicable | 2 May 2022 |
| NCT03160599 | Restricted Calorie Ketogenic Diet as a Treatment in Malignant Tumors | Malignant Tumors | ketogenic diet | Adverse events of patients on high-fat diet | Not Applicable | 5 September 2018 |
| NCT02286167 | Glioma Modified Atkins-based Diet in Patients With Glioblastoma | Glioblastoma Multiforme | Diet modification | Feasibility of intermittent modified Atkins diet in patients with GBM assessed by percent of patients able to remain on the diet and achieve nutritional goals|Biologic activity measured by pre- and post-study cerebral glutamate and glutamine concentrations assessed by MRS|Tolerability assessed by percent of patients who have an adverse reaction of any grade attributed to the diet of possible, probable, or definite|Dietary Activity | Not Applicable | 12 May 2020 |
5. Nanocarrier Treatment Options
5.1. Liposomes
5.2. Polymer Micelles
5.3. Magnetic Nanoparticles
5.4. Other Nano-Complex Applications
5.5. Nanocarriers in Clinical Trials for the Treatment of GBM
6. Future Treatment Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weller, M.; Wick, W.; Aldape, K.; Brada, M.; Berger, M.; Pfister, S.M.; Nishikawa, R.; Rosenthal, M.; Wen, P.Y.; Stupp, R. Glioma. Nat. Rev. Dis. Prim. 2015, 1, 15017. [Google Scholar] [CrossRef]
- Onishi, S.; Yamasaki, F.; Amatya, V.J.; Takayasu, T.; Yonezawa, U.; Taguchi, A.; Ohba, S.; Takeshima, Y.; Horie, N.; Sugiyama, K. Characteristics and therapeutic strategies of radiation-induced glioma: Case series and comprehensive literature review. J. Neuro-Oncol. 2022, 159, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Bian, W.; Khayal, I.S.; Lupo, J.M.; McGue, C.; Vandenberg, S.; Lamborn, K.R.; Chang, S.M.; Cha, S.; Nelson, S.J. Multiparametric characterization of grade 2 glioma subtypes using magnetic resonance spectroscopic, perfusion, and diffusion imaging. Transl. Oncol. 2009, 2, 271–280. [Google Scholar] [CrossRef] [Green Version]
- Jo, J.; Schiff, D. Current Considerations in the Treatment of Grade 3 Gliomas. Curr. Treat. Options Oncol. 2022, 23, 1219–1232. [Google Scholar] [CrossRef] [PubMed]
- Mair, M.J.; Geurts, M.; van den Bent, M.J.; Berghoff, A.S. A basic review on systemic treatment options in WHO grade II-III gliomas. Cancer Treat. Rev. 2021, 92, 102124. [Google Scholar] [CrossRef] [PubMed]
- Perez, A.; Huse, J.T. The Evolving Classification of Diffuse Gliomas: World Health Organization Updates for 2021. Curr. Neurol. Neurosci. Rep. 2021, 21, 67. [Google Scholar] [CrossRef]
- Taylor, O.G.; Brzozowski, J.S.; Skelding, K.A. Glioblastoma multiforme: An overview of emerging therapeutic targets. Front. Oncol. 2019, 9, 963. [Google Scholar] [CrossRef] [Green Version]
- Grochans, S.; Cybulska, A.M.; Simińska, D.; Korbecki, J.; Kojder, K.; Chlubek, D.; Baranowska-Bosiacka, I. Epidemiology of Glioblastoma Multiforme–Literature Review. Cancers 2022, 14, 2412. [Google Scholar] [CrossRef]
- Simińska, D.; Korbecki, J.; Kojder, K.; Kapczuk, P.; Fabiańska, M.; Gutowska, I.; Machoy-Mokrzyńska, A.; Chlubek, D.; Baranowska-Bosiacka, I. Epidemiology of anthropometric factors in glioblastoma multiforme—Literature review. Brain Sci. 2021, 11, 116. [Google Scholar] [CrossRef]
- Kita, D.; Ciernik, I.F.; Vaccarella, S.; Franceschi, S.; Kleihues, P.; Lütolf, U.M.; Ohgaki, H. Age as a predictive factor in glioblastomas: Population-based study. Neuroepidemiology 2009, 33, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Grunert, M.; Kassubek, R.; Danz, B.; Klemenz, B.; Hasslacher, S.; Stroh, S.; Schneele, L.; Langhans, J.; Ströbele, S.; Barry, S.E. Radiation and brain tumors: An overview. Crit. Rev. Oncog. 2018, 23, 119–138. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, V.; Rabiee, A.; Khademi, F. Glioblastoma multiforme: A glance at advanced therapies based on nanotechnology. J. Chemother. 2020, 32, 107–117. [Google Scholar] [CrossRef]
- Kabat, G.C.; Etgen, A.M.; Rohan, T.E. Do Steroid Hormones Play a Role in the Etiology of Glioma? Steroid Hormones and Glioma. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2421–2427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamimi, A.F.; Juweid, M. Epidemiology and outcome of glioblastoma. In Glioblastoma; De Vleeschouwer, S., Ed.; Exon Publications: Brisbane, Australia, 2017; pp. 143–153. [Google Scholar]
- Alifieris, C.; Trafalis, D.T. Glioblastoma multiforme: Pathogenesis and treatment. Pharmacol. Ther. 2015, 152, 63–82. [Google Scholar] [CrossRef] [PubMed]
- Hanif, F.; Muzaffar, K.; Perveen, K.; Malhi, S.M.; Simjee, S.U. Glioblastoma multiforme: A review of its epidemiology and pathogenesis through clinical presentation and treatment. Asian Pac. J. Cancer Prev. APJCP 2017, 18, 3–9. [Google Scholar] [PubMed]
- Brat, D.J.; Perry, A. Astrocytic and oligodendroglial tumors. In Practical Surgical Neuropathology: A Diagnostic Approach; Elsevier: Amsterdam, The Netherlands, 2018; pp. 91–123. [Google Scholar]
- Azagury, D.E.; Dua, M.M.; Barrese, J.C.; Henderson, J.M.; Buchs, N.C.; Ris, F.; Cloyd, J.M.; Martinie, J.B.; Razzaque, S.; Nicolau, S.J. Image-guided surgery. Curr. Probl. Surg. 2015, 52, 476–520. [Google Scholar] [CrossRef] [Green Version]
- Watts, C.; Sanai, N. Surgical approaches for the gliomas. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2016; Volume 134, pp. 51–69. [Google Scholar]
- Oszvald, Á.; Güresir, E.; Setzer, M.; Vatter, H.; Senft, C.; Seifert, V.; Franz, K. Glioblastoma therapy in the elderly and the importance of the extent of resection regardless of age. J. Neurosurg. 2012, 116, 357–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kircher, M.F.; De La Zerda, A.; Jokerst, J.V.; Zavaleta, C.L.; Kempen, P.J.; Mittra, E.; Pitter, K.; Huang, R.; Campos, C.; Habte, F.J. A brain tumor molecular imaging strategy using a new triple-modality MRI-photoacoustic-Raman nanoparticle. Nat. Med. 2012, 18, 829–834. [Google Scholar] [CrossRef]
- Klimberg, V.S.; Rivere, A. Ultrasound image-guided core biopsy of the breast. Chin. Clin. Oncol. 2016, 5, 33. [Google Scholar] [CrossRef] [Green Version]
- Jenkinson, M.D.; Barone, D.G.; Bryant, A.; Vale, L.; Bulbeck, H.; Lawrie, T.A.; Hart, M.G.; Watts, C.J. Intraoperative imaging technology to maximise extent of resection for glioma. Cochrane Database Syst. Rev. 2018, 1, CD012788. [Google Scholar] [CrossRef]
- Pan, J.W.; Moon, C.H.; Hetherington, H. Cerebrospinal fluid–suppressed T2-weighted MR imaging at 7 T for human brain. Magn. Reson. Med. 2019, 81, 2924–2936. [Google Scholar] [CrossRef] [PubMed]
- Gerard, I.J.; Kersten-Oertel, M.; Petrecca, K.; Sirhan, D.; Hall, J.A.; Collins, D.L.J. Brain shift in neuronavigation of brain tumors: A review. Med. Image Anal. 2017, 35, 403–420. [Google Scholar] [CrossRef] [PubMed]
- Diaz, R.J.; Dios, R.R.; Hattab, E.M.; Burrell, K.; Rakopoulos, P.; Sabha, N.; Hawkins, C.; Zadeh, G.; Rutka, J.T.; Cohen-Gadol, A.A. Study of the biodistribution of fluorescein in glioma-infiltrated mouse brain and histopathological correlation of intraoperative findings in high-grade gliomas resected under fluorescein fluorescence guidance. J. Neurosurg. 2015, 122, 1360–1369. [Google Scholar] [CrossRef] [Green Version]
- Maragkos, G.A.; Schüpper, A.J.; Lakomkin, N.; Sideras, P.; Price, G.; Baron, R.; Hamilton, T.; Haider, S.; Lee, I.Y.; Hadjipanayis, C.G. Fluorescence-guided high-grade glioma surgery more than four hours after 5-aminolevulinic acid administration. Front. Neurol. 2021, 12, 644804. [Google Scholar] [CrossRef] [PubMed]
- Nimsky, C.; Ganslandt, O.; Hastreiter, P.; Fahlbusch, R. Intraoperative compensation for brain shift. Surg. Neurol. 2001, 56, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Iversen, D.H.; Wein, W.; Lindseth, F.; Unsgård, G.; Reinertsen, I.J. Automatic intraoperative correction of brain shift for accurate neuronavigation. World Neurosurg. 2018, 120, e1071–e1078. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; Van Den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [Green Version]
- Syro, L.V.; Rotondo, F.; Camargo, M.; Ortiz, L.D.; Serna, C.A.; Kovacs, K. Temozolomide and pituitary tumors: Current understanding, unresolved issues, and future directions. Front. Endocrinol. 2018, 9, 318. [Google Scholar] [CrossRef] [Green Version]
- Aasland, D.; Götzinger, L.; Hauck, L.; Berte, N.; Meyer, J.; Effenberger, M.; Schneider, S.; Reuber, E.E.; Roos, W.P.; Tomicic, M.T. Temozolomide Induces Senescence and Repression of DNA Repair Pathways in Glioblastoma Cells via Activation of ATR–CHK1, p21, and NF-κBMechanisms of Methylating Anticancer Drug–Induced Senescence. Cancer Res. 2019, 79, 99–113. [Google Scholar] [CrossRef] [Green Version]
- Krauze, A.V.; Myrehaug, S.D.; Chang, M.G.; Holdford, D.J.; Smith, S.; Shih, J.; Tofilon, P.J.; Fine, H.A.; Camphausen, K. A phase 2 study of concurrent radiation therapy, temozolomide, and the histone deacetylase inhibitor valproic acid for patients with glioblastoma. Int. J. Radiat. Oncol. 2015, 92, 986–992. [Google Scholar] [CrossRef] [Green Version]
- Lu, V.M.; Jue, T.R.; McDonald, K. Cytotoxic lanthanum oxide nanoparticles sensitize glioblastoma cells to radiation therapy and temozolomide: An in vitro rationale for translational studies. Sci. Rep. 2020, 10, 18156. [Google Scholar] [CrossRef]
- Le Rhun, E.; Devos, P.; Houillier, C.; Cartalat, S.; Chinot, O.; Di Stefano, A.L.; Lepage, C.; Reyns, N.; Dubois, F.; Weller, M. Romiplostim for temozolomide-induced thrombocytopenia in glioblastoma: The PLATUM trial. Neurology 2019, 93, e1799–e1806. [Google Scholar] [CrossRef] [PubMed]
- Kaina, B.; Christmann, M. DNA repair in personalized brain cancer therapy with temozolomide and nitrosoureas. DNA Repair 2019, 78, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Kleiner, L.W.; Wright, J.C.; Wang, Y. Evolution of implantable and insertable drug delivery systems. J. Control. Release 2014, 181, 1–10. [Google Scholar] [CrossRef]
- Wu, W.; Klockow, J.L.; Zhang, M.; Lafortune, F.; Chang, E.; Jin, L.; Wu, Y.; Daldrup-Link, H.E. Glioblastoma multiforme (GBM): An overview of current therapies and mechanisms of resistance. Pharmacol. Res. 2021, 171, 105780. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, T.F.; Sosman, J.; Gerson, S.L.; Liu, L.; Dolan, E.; Lin, S.; Vokes, E.E. Phase II trial of the O 6-alkylguanine DNA alkyltransferase inhibitor O 6-benzylguanine and 1, 3-bis (2-chloroethyl)-1-nitrosourea in advanced melanoma. Clin. Cancer Res. 2005, 11, 7861–7865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bock, H.C.; Puchner, M.J.A.; Lohmann, F.; Schütze, M.; Koll, S.; Ketter, R.; Buchalla, R.; Rainov, N.; Kantelhardt, S.R.; Rohde, V. First-line treatment of malignant glioma with carmustine implants followed by concomitant radiochemotherapy: A multicenter experience. Neurosurg. Rev. 2010, 33, 441–449. [Google Scholar] [CrossRef] [Green Version]
- Lombardi, G.; De Salvo, G.L.; Brandes, A.A.; Eoli, M.; Rudà, R.; Faedi, M.; Lolli, I.; Pace, A.; Daniele, B.; Pasqualetti, F. Regorafenib compared with lomustine in patients with relapsed glioblastoma (REGOMA): A multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 2019, 20, 110–119. [Google Scholar] [CrossRef]
- Germano, I.M.; Ziu, M.; Wen, P.; Ormond, D.R.; Olson, J. Congress of Neurological Surgeons systematic review and evidence-based guidelines update on the role of cytotoxic chemotherapy and other cytotoxic therapies in the management of progressive glioblastoma in adults. J. Neuro-Oncol. 2022, 158, 225–253. [Google Scholar] [CrossRef]
- Brandes, A.A.; Finocchiaro, G.; Zagonel, V.; Reni, M.; Caserta, C.; Fabi, A.; Clavarezza, M.; Maiello, E.; Eoli, M.; Lombardi, G. AVAREG: A phase II, randomized, noncomparative study of fotemustine or bevacizumab for patients with recurrent glioblastoma. Neuro-Oncology 2016, 18, 1304–1312. [Google Scholar] [CrossRef] [Green Version]
- Ameratunga, M.; Pavlakis, N.; Wheeler, H.; Grant, R.; Simes, J.; Khasraw, M. Anti-angiogenic therapy for high-grade glioma. Cochrane Database Syst. Rev. 2018, 1, CD008218. [Google Scholar] [CrossRef]
- Diaz, R.J.; Ali, S.; Qadir, M.G.; De La Fuente, M.I.; Ivan, M.E.; Komotar, R. The role of bevacizumab in the treatment of glioblastoma. J. Neuro-Oncol. 2017, 133, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, A.R.; Kirkpatrick, J.P.; Fiveash, J.B.; Shih, H.A.; Koay, E.J.; Lutz, S.; Petit, J.; Chao, S.T.; Brown, P.D.; Vogelbaum, M. Radiation therapy for glioblastoma: Executive summary of an American Society for Radiation Oncology evidence-based clinical practice guideline. Pr. Radiat. Oncol. 2016, 6, 217–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murshed, H. Fundamentals of Radiation Oncology: Physical, Biological, and Clinical Aspects; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar]
- Liu, P.; Liu, G.; Wang, G.; Zhou, W.; Sun, Y.; Chen, W.; Zeng, Q.; Hong, J.; Xie, Q.; Ou, L. Comparison of dosimetric gains provided by intensity-modulated radiotherapy, volume-modulated arc therapy, and helical tomotherapy for high-grade glioma. BioMed Res. Int. 2020, 2020, 4258989. [Google Scholar] [CrossRef] [Green Version]
- Ganz, J.C. The journey from proton to gamma knife. Prog. Brain Res. 2014, 215, 67–75. [Google Scholar] [PubMed]
- Leber, K.A.; Berglöff, J.; Pendl, G.J. Dose—Response tolerance of the visual pathways and cranial nerves of the cavernous sinus to stereotactic radiosurgery. J. Neurosurg. 1998, 88, 43–50. [Google Scholar] [CrossRef]
- Redmond, K.J.; Mehta, M.J.C. Stereotactic radiosurgery for glioblastoma. Cureus 2015, 7, e413. [Google Scholar] [CrossRef] [Green Version]
- Barbarite, E.; Sick, J.T.; Berchmans, E.; Bregy, A.; Shah, A.H.; Elsayyad, N.; Komotar, R. The role of brachytherapy in the treatment of glioblastoma multiforme. Neurosurg. Rev. 2017, 40, 195–211. [Google Scholar] [CrossRef]
- Parvez, K.; Parvez, A.; Zadeh, G.J. The diagnosis and treatment of pseudoprogression, radiation necrosis and brain tumor recurrence. Int. J. Mol. Sci. 2014, 15, 11832–11846. [Google Scholar] [CrossRef] [Green Version]
- Kong, L.; Wu, J.; Gao, J.; Qiu, X.; Yang, J.; Hu, J.; Hu, W.; Mao, Y.; Lu, J. Particle radiation therapy in the management of malignant glioma: Early experience at the Shanghai Proton and Heavy Ion Center. Cancer 2020, 126, 2802–2810. [Google Scholar] [CrossRef]
- Rockwell, S.; Dobrucki, I.T.; Kim, E.Y.; Marrison, S.T.; Vu, V.T. Hypoxia and radiation therapy: Past history, ongoing research, and future promise. Curr. Mol. Med. 2009, 9, 442–458. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Wei, D.; Dai, X.; Stevens, M.F.; Bradshaw, T.D.; Luo, Y.; Zhang, J. C8-substituted imidazotetrazine analogs overcome temozolomide resistance by inducing DNA adducts and DNA damage. Front. Oncol. 2019, 9, 485. [Google Scholar] [CrossRef]
- Verbeek, B.; Southgate, T.D.; Gilham, D.E.; Margison, G.P. O6-Methylguanine-DNA methyltransferase inactivation and chemotherapy. Br. Med. Bull. 2008, 85, 17–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thon, N.; Kreth, S.; Kreth, F.-W. Personalized treatment strategies in glioblastoma: MGMT promoter methylation status. OncoTargets Ther. 2013, 6, 1363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szopa, W.; Burley, T.A.; Kramer-Marek, G.; Kaspera, W. Diagnostic and therapeutic biomarkers in glioblastoma: Current status and future perspectives. BioMed Res. Int. 2017, 2017, 8013575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cabrini, G.; Fabbri, E.; Lo Nigro, C.; Dechecchi, M.C.; Gambari, R. Regulation of expression of O6-methylguanine-DNA methyltransferase and the treatment of glioblastoma. Int. J. Oncol. 2015, 47, 417–428. [Google Scholar] [CrossRef] [Green Version]
- Erasimus, H.; Gobin, M.; Niclou, S.; Van Dyck, E. DNA repair mechanisms and their clinical impact in glioblastoma. Mutat. Res. Mol. Mech. Mutagen. 2016, 769, 19–35. [Google Scholar] [CrossRef]
- Stritzelberger, J.; Distel, L.; Buslei, R.; Fietkau, R.; Putz, F. Acquired temozolomide resistance in human glioblastoma cell line U251 is caused by mismatch repair deficiency and can be overcome by lomustine. Clin. Transl. Oncol. 2018, 20, 508–516. [Google Scholar] [CrossRef]
- Dirkse, A.; Golebiewska, A.; Buder, T.; Nazarov, P.V.; Muller, A.; Poovathingal, S.; Brons, N.H.; Leite, S.; Sauvageot, N.; Sarkisjan, D. Stem cell-associated heterogeneity in Glioblastoma results from intrinsic tumor plasticity shaped by the microenvironment. Nat. Commun. 2019, 10, 1787. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.K.; Hawkins, C.; Clarke, I.D.; Squire, J.A.; Bayani, J.; Hide, T.; Henkelman, R.M.; Cusimano, M.D.; Dirks, P.B. Identification of human brain tumour initiating cells. Nature 2004, 432, 396–401. [Google Scholar] [CrossRef]
- Si, D.; Yin, F.; Peng, J.; Zhang, G. High expression of CD44 predicts a poor prognosis in glioblastomas. Cancer Manag. Res. 2020, 12, 769. [Google Scholar] [CrossRef] [Green Version]
- Bao, S.; Wu, Q.; McLendon, R.E.; Hao, Y.; Shi, Q.; Hjelmeland, A.B.; Dewhirst, M.W.; Bigner, D.D.; Rich, J.N. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 2006, 444, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Uribe, D.; Torres, A.; Rocha, J.D.; Niechi, I.; Oyarzún, C.; Sobrevia, L.; San Martín, R.; Quezada, C. Multidrug resistance in glioblastoma stem-like cells: Role of the hypoxic microenvironment and adenosine signaling. Mol. Asp. Med. 2017, 55, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Westover, D.; Li, F.J. New trends for overcoming ABCG2/BCRP-mediated resistance to cancer therapies. J. Exp. Clin. Cancer Res. 2015, 34, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolenda, J.; Jensen, S.S.; Aaberg-Jessen, C.; Christensen, K.; Andersen, C.; Brünner, N.; Kristensen, B.W. Effects of hypoxia on expression of a panel of stem cell and chemoresistance markers in glioblastoma-derived spheroids. J. Neuro-Oncol. 2011, 103, 43–58. [Google Scholar] [CrossRef]
- Walsh, J.C.; Lebedev, A.; Aten, E.; Madsen, K.; Marciano, L.; Kolb, H.C. The clinical importance of assessing tumor hypoxia: Relationship of tumor hypoxia to prognosis and therapeutic opportunities. Antioxid. Redox Signal. 2014, 21, 1516–1554. [Google Scholar] [CrossRef]
- Monteiro, A.R.; Hill, R.; Pilkington, G.J.; Madureira, P.A. The role of hypoxia in glioblastoma invasion. Cells 2017, 6, 45. [Google Scholar] [CrossRef] [Green Version]
- Logun, M.; Zhao, W.; Mao, L.; Karumbaiah, L. Microfluidics in malignant glioma research and precision medicine. Adv. Biosyst. 2018, 2, 1700221. [Google Scholar] [CrossRef]
- Hewitt, G.; Korolchuk, V.I. Repair, reuse, recycle: The expanding role of autophagy in genome maintenance. Trends Cell Biol. 2017, 27, 340–351. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.-L.; DeLay, M.; Jahangiri, A.; Molinaro, A.M.; Rose, S.D.; Carbonell, W.S.; Aghi, M.K. Hypoxia-Induced Autophagy Promotes Tumor Cell Survival and Adaptation to Antiangiogenic Treatment in GlioblastomaAutophagy Mediates Resistance to Antiangiogenic Therapy. Cancer Res. 2012, 72, 1773–1783. [Google Scholar] [CrossRef] [Green Version]
- Sotelo, J.; Briceño, E.; López-González, M.A. Adding chloroquine to conventional treatment for glioblastoma multiforme: A randomized, double-blind, placebo-controlled trial. Ann. Intern. Med. 2006, 144, 337–343. [Google Scholar] [CrossRef]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef]
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef]
- Keaney, J.; Campbell, M. The dynamic blood-brain barrier. FEBS J. 2015, 282, 4067–4079. [Google Scholar] [CrossRef] [PubMed]
- Mergenthaler, P.; Lindauer, U.; Dienel, G.A.; Meisel, A. Sugar for the brain: The role of glucose in physiological and pathological brain function. Trends Neurosci. 2013, 36, 587–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hottinger, A.F.; Stupp, R.; Homicsko, K. Standards of care and novel approaches in the management of glioblastoma multiforme. Chin. J. Cancer 2014, 33, 32–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barani, I.J.; Larson, D.A. Radiation therapy of glioblastoma. Curr. Underst. Treat. Gliomas 2015, 163, 49–73. [Google Scholar]
- Schneider, S.W.; Ludwig, T.; Tatenhorst, L.; Braune, S.; Oberleithner, H.; Senner, V.; Paulus, W. Glioblastoma cells release factors that disrupt blood-brain barrier features. Acta Neuropathol. 2004, 107, 272–276. [Google Scholar] [CrossRef]
- Ishihara, H.; Kubota, H.; Lindberg, R.L.; Leppert, D.; Gloor, S.M.; Errede, M.; Virgintino, D.; Fontana, A.; Yonekawa, Y.; Frei, K. Endothelial cell barrier impairment induced by glioblastomas and transforming growth factor beta2 involves matrix metalloproteinases and tight junction proteins. J. Neuropathol. Exp. Neurol. 2008, 67, 435–448. [Google Scholar] [CrossRef] [Green Version]
- Dubois, L.G.; Campanati, L.; Righy, C.; D’Andrea-Meira, I.; Spohr, T.C.; Porto-Carreiro, I.; Pereira, C.M.; Balça-Silva, J.; Kahn, S.A.; DosSantos, M.F.; et al. Gliomas and the vascular fragility of the blood brain barrier. Front. Cell Neurosci. 2014, 8, 418. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Xu, C.L.; Liu, C.M. Drug delivery strategies to enhance the permeability of the blood-brain barrier for treatment of glioma. Drug Des. Dev. Ther. 2015, 9, 2089–2100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lemée, J.M.; Clavreul, A.; Menei, P. Intratumoral heterogeneity in glioblastoma: Don’t forget the peritumoral brain zone. Neuro-Oncology 2015, 17, 1322–1332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oberoi, R.K.; Parrish, K.E.; Sio, T.T.; Mittapalli, R.K.; Elmquist, W.F.; Sarkaria, J.N. Strategies to improve delivery of anticancer drugs across the blood-brain barrier to treat glioblastoma. Neuro-Oncology 2016, 18, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Pafundi, D.H.; Laack, N.N.; Youland, R.S.; Parney, I.F.; Lowe, V.J.; Giannini, C.; Kemp, B.J.; Grams, M.P.; Morris, J.M.; Hoover, J.M.; et al. Biopsy validation of 18F-DOPA PET and biodistribution in gliomas for neurosurgical planning and radiotherapy target delineation: Results of a prospective pilot study. Neuro-Oncology 2013, 15, 1058–1067. [Google Scholar] [CrossRef]
- Juillerat-Jeanneret, L. The targeted delivery of cancer drugs across the blood-brain barrier: Chemical modifications of drugs or drug-nanoparticles? Drug Discov. Today 2008, 13, 1099–1106. [Google Scholar] [CrossRef]
- Liu, H.-J.; Xu, P. Strategies to overcome/penetrate the BBB for systemic nanoparticle delivery to the brain/brain tumor. Adv. Drug Deliv. Rev. 2022, 191, 114619. [Google Scholar] [CrossRef]
- Tzeng, S.Y.; Green, J.J. Therapeutic nanomedicine for brain cancer. Ther. Deliv. 2013, 4, 687–704. [Google Scholar] [CrossRef] [Green Version]
- Hsu, J.-F.; Chu, S.-M.; Liao, C.-C.; Wang, C.-J.; Wang, Y.-S.; Lai, M.-Y.; Wang, H.-C.; Huang, H.-R.; Tsai, M.-H. Nanotechnology and nanocarrier-based drug delivery as the potential therapeutic strategy for glioblastoma multiforme: An update. Cancers 2021, 13, 195. [Google Scholar] [CrossRef] [PubMed]
- Beier, D.; Schulz, J.B.; Beier, C.P. Chemoresistance of glioblastoma cancer stem cells-much more complex than expected. Mol. Cancer 2011, 10, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, J.-F.; Chu, S.-M.; Liao, C.-C.; Wang, C.-J.; Wang, Y.-S.; Lai, M.-Y.; Wang, H.-C.; Huang, H.-R.; Tsai, M.-H. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 2006, 66, 7843–7848. [Google Scholar]
- Sundar, S.J.; Hsieh, J.K.; Manjila, S.; Lathia, J.D.; Sloan, A. The role of cancer stem cells in glioblastoma. Neurosurg. Focus 2014, 37, E6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gimple, R.C.; Bhargava, S.; Dixit, D.; Rich, J.N. Glioblastoma stem cells: Lessons from the tumor hierarchy in a lethal cancer. Genes Dev. 2019, 33, 591–609. [Google Scholar] [CrossRef]
- Singh, S.K.; Clarke, I.D.; Terasaki, M.; Bonn, V.E.; Hawkins, C.; Squire, J.; Dirks, P.B. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003, 63, 5821–5828. [Google Scholar] [PubMed]
- Stupp, R.; Hegi, M.E.; Mason, W.P.; van den Bent, M.J.; Taphoorn, M.J.; Janzer, R.C.; Ludwin, S.K.; Allgeier, A.; Fisher, B.; Belanger, K.; et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009, 10, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; van Straten, D.; Broekman, M.L.D.; Préat, V.; Schiffelers, R.M. Nanocarrier-based drug combination therapy for glioblastoma. Theranostics 2020, 10, 1355–1372. [Google Scholar] [CrossRef]
- Aldape, K.; Zadeh, G.; Mansouri, S.; Reifenberger, G.; von Deimling, A. Glioblastoma: Pathology, molecular mechanisms and markers. Acta Neuropathol. 2015, 129, 829–848. [Google Scholar] [CrossRef] [PubMed]
- Charles, N.A.; Holland, E.C.; Gilbertson, R.; Glass, R.; Kettenmann, H. The brain tumor microenvironment. Glia 2011, 59, 1169–1180. [Google Scholar] [CrossRef]
- Martini, M.; Vecchione, L.; Siena, S.; Tejpar, S.; Bardelli, A. Targeted therapies: How personal should we go? Nat. Rev. Clin. Oncol. 2012, 9, 87–97. [Google Scholar] [CrossRef]
- Martini, M.; Vecchione, L.; Siena, S.; Tejpar, S.; Bardelli, A. Extracellular vesicle communication pathways as regulatory targets of oncogenic transformation. In Seminars in Cell & Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Vasconcelos, M.H.; Caires, H.R.; Ābols, A.; Xavier, C.P.; Linē, A. Extracellular vesicles as a novel source of biomarkers in liquid biopsies for monitoring cancer progression and drug resistance. Drug Resist. Updates 2019, 47, 100647. [Google Scholar] [CrossRef]
- Bach, D.H.; Hong, J.Y.; Park, H.J.; Lee, S.K. The role of exosomes and miRNAs in drug-resistance of cancer cells. Int. J. Cancer 2017, 141, 220–230. [Google Scholar] [CrossRef] [Green Version]
- Yekula, A.; Yekula, A.; Muralidharan, K.; Kang, K.; Carter, B.S.; Balaj, L. Extracellular vesicles in glioblastoma tumor microenvironment. Front. Immunol. 2020, 10, 3137. [Google Scholar] [CrossRef] [PubMed]
- Samuel, P.; Fabbri, M.; Carter, D.R.F. Mechanisms of drug resistance in cancer: The role of extracellular vesicles. Proteomics 2017, 17, 1600375. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, J.P.; Stevens, M.M. Strategic design of extracellular vesicle drug delivery systems. Adv. Drug Deliv. Rev. 2018, 130, 12–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, H.; Gu, J.; Zhang, J.; Shi, H.; Qian, H.; Wang, D.; Xu, W.; Pan, J.; Santos, H.A. Engineered extracellular vesicles for cancer therapy. Adv. Mater. 2021, 33, 2005709. [Google Scholar] [CrossRef] [PubMed]
- Esfahani, K.; Roudaia, L.; Buhlaiga, N.; Del Rincon, S.; Papneja, N.; Miller, W. A review of cancer immunotherapy: From the past, to the present, to the future. Curr. Oncol. 2020, 27, 87–97. [Google Scholar] [CrossRef]
- Huang, B.; Li, X.; Li, Y.; Zhang, J.; Zong, Z.; Zhang, H. Current immunotherapies for glioblastoma multiforme. Front. Immunol. 2021, 11, 603911. [Google Scholar] [CrossRef]
- Khawli, L.A.; Hu, P.; Epstein, A.L. Cytokine, chemokine, and co-stimulatory fusion proteins for the immunotherapy of solid tumors. In Therapeutic Antibodies; Handbook of Experimental Pharmacology Book Series; Springer: Berlin/Heidelberg, Germany, 2008; Volume 181, pp. 291–328. [Google Scholar]
- Kominsky, S.L.; Subramaniam, P.S.; Johnson, H.M.; Torres, B.A. Inhibitory effects of IFN-gamma and acyclovir on the glioblastoma cell cycle. J. Interferon Cytokine Res. 2000, 20, 463–469. [Google Scholar] [CrossRef]
- Weiss, T.; Puca, E.; Silginer, M.; Hemmerle, T.; Pazahr, S.; Bink, A.; Weller, M.; Neri, D.; Roth, P. Immunocytokines are a promising immunotherapeutic approach against glioblastoma. Sci. Transl. Med. 2020, 12, eabb2311. [Google Scholar] [CrossRef]
- Sönmez, C.; Wölfer, J.; Holling, M.; Brokinkel, B.; Stummer, W.; Wiendl, H.; Thomas, C.; Schulte-Mecklenbeck, A.; Grauer, O.M. Blockade of inhibitory killer cell immunoglobulin-like receptors and IL-2 triggering reverses the functional hypoactivity of tumor-derived NK-cells in glioblastomas. Sci. Rep. 2022, 12, 6769. [Google Scholar] [CrossRef]
- Happold, C.; Roth, P.; Silginer, M.; Florea, A.M.; Lamszus, K.; Frei, K.; Deenen, R.; Reifenberger, G.; Weller, M. Interferon-β induces loss of spherogenicity and overcomes therapy resistance of glioblastoma stem cells. Mol. Cancer Ther. 2014, 13, 948–961. [Google Scholar] [CrossRef] [Green Version]
- Birocchi, F.; Cusimano, M.; Rossari, F.; Beretta, S.; Rancoita, P.M.V.; Ranghetti, A.; Colombo, S.; Costa, B.; Angel, P.; Sanvito, F.; et al. Targeted inducible delivery of immunoactivating cytokines reprograms glioblastoma microenvironment and inhibits growth in mouse models. Sci. Transl. Med. 2022, 14, eabl4106. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.A.; Dudley, M.E.; Wunderlich, J.R.; Hughes, M.S.; Yang, J.C.; Sherry, R.M.; Royal, R.E.; Topalian, S.L.; Kammula, U.S.; Restifo, N.P.; et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 2006, 314, 126–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borghaei, H.; Smith, M.R.; Campbell, K.S. Immunotherapy of cancer. Eur. J. Pharmacol. 2009, 625, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Narita, Y. Bevacizumab for glioblastoma. Ther. Clin. Risk Manag. 2015, 11, 1759–1765. [Google Scholar] [CrossRef] [Green Version]
- Hasselbalch, B.; Lassen, U.; Hansen, S.; Holmberg, M.; Sørensen, M.; Kosteljanetz, M.; Broholm, H.; Stockhausen, M.T.; Poulsen, H.S. Cetuximab, bevacizumab, and irinotecan for patients with primary glioblastoma and progression after radiation therapy and temozolomide: A phase II trial. Neuro-Oncology 2010, 12, 508–516. [Google Scholar]
- McCrea, H.J.; Ivanidze, J.; O’Connor, A.; Hersh, E.H.; Boockvar, J.A.; Gobin, Y.P.; Knopman, J.; Greenfield, J.P. Intraarterial delivery of bevacizumab and cetuximab utilizing blood-brain barrier disruption in children with high-grade glioma and diffuse intrinsic pontine glioma: Results of a phase I trial. J. Neurosurg. Pediatr. 2021, 28, 371–379. [Google Scholar] [CrossRef]
- Kochar, A.S.; Madhavan, M.; Manjila, S.; Scoco, A.; Belle, V.K.; Geertman, R.T. Contemporary Updates on Clinical Trials of Antiangiogenic Agents in the Treatment of Glioblastoma Multiforme. Asian J. Neurosurg. 2018, 13, 546–554. [Google Scholar] [CrossRef]
- Lukas, R.V.; Rodon, J.; Becker, K.; Wong, E.T.; Shih, K.; Touat, M.; Fassò, M.; Osborne, S.; Molinero, L.; O’Hear, C.; et al. Clinical activity and safety of atezolizumab in patients with recurrent glioblastoma. J. Neurooncol. 2018, 140, 317–328. [Google Scholar] [CrossRef]
- Nayak, L.; Molinaro, A.M.; Peters, K.; Clarke, J.L.; Jordan, J.T.; de Groot, J.; Nghiemphu, L.; Kaley, T.; Colman, H.; McCluskey, C.; et al. Randomized Phase II and Biomarker Study of Pembrolizumab plus Bevacizumab versus Pembrolizumab Alone for Patients with Recurrent Glioblastoma. Clin. Cancer Res. 2021, 27, 1048–1057. [Google Scholar] [CrossRef]
- Hong, M.; Clubb, J.D.; Chen, Y.Y. Engineering CAR-T cells for next-generation cancer therapy. Cancer Cell 2020, 38, 473–488. [Google Scholar] [CrossRef]
- Soler, D.C.; Kerstetter-Fogle, A.; McCormick, T.S.; Sloan, A.E. Using chimeric antigen receptor T-cell therapy to fight glioblastoma multiforme: Past, present and future developments. J. Neuro-Oncol. 2021, 156, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Sampson, J.H.; Choi, B.D.; Sanchez-Perez, L.; Suryadevara, C.M.; Snyder, D.J.; Flores, C.T.; Schmittling, R.J.; Nair, S.K.; Reap, E.A.; Norberg, P.K. EGFRvIII mCAR-Modified T-Cell Therapy Cures Mice with Established Intracerebral Glioma and Generates Host Immunity against Tumor-Antigen LossEGFRvIII mCARs for Malignant Glioma. Clin. Cancer Res. 2014, 20, 972–984. [Google Scholar] [CrossRef] [Green Version]
- Choi, B.D.; Yu, X.; Castano, A.P.; Bouffard, A.A.; Schmidts, A.; Larson, R.C.; Bailey, S.R.; Boroughs, A.C.; Frigault, M.J.; Leick, M.B. CAR-T cells secreting BiTEs circumvent antigen escape without detectable toxicity. Nat. Biotechnol. 2019, 37, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Baeuerle, P.A.; Ding, J.; Patel, E.; Thorausch, N.; Horton, H.; Gierut, J.; Scarfo, I.; Choudhary, R.; Kiner, O.; Krishnamurthy, J. Synthetic TRuC receptors engaging the complete T cell receptor for potent anti-tumor response. Nat. Commun. 2019, 10, 2087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roybal, K.T.; Williams, J.Z.; Morsut, L.; Rupp, L.J.; Kolinko, I.; Choe, J.H.; Walker, W.J.; McNally, K.A.; Lim, W.A. Engineering T cells with customized therapeutic response programs using synthetic notch receptors. Cell 2016, 167, 419–432.e16. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Starr, R.; Chang, W.-C.; Aguilar, B.; Alizadeh, D.; Wright, S.L.; Yang, X.; Brito, A.; Sarkissian, A.; Ostberg, J.R. Chlorotoxin-directed CAR T cells for specific and effective targeting of glioblastoma. Sci. Transl. Med. 2020, 12, eaaw2672. [Google Scholar] [CrossRef]
- Jin, L.; Ge, H.; Long, Y.; Yang, C.; Chang, Y.; Mu, L.; Sayour, E.J.; De Leon, G.; Wang, Q.J.; Yang, J.C. CD70, a novel target of CAR T-cell therapy for gliomas. Neuro-Oncology 2018, 20, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Bielamowicz, K.; Fousek, K.; Byrd, T.T.; Samaha, H.; Mukherjee, M.; Aware, N.; Wu, M.-F.; Orange, J.S.; Sumazin, P.; Man, T.-K. Trivalent CAR T cells overcome interpatient antigenic variability in glioblastoma. Neuro-Oncology 2018, 20, 506–518. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, H.M.; Saha, D. The Current State of Oncolytic Herpes Simplex Virus for Glioblastoma Treatment. Oncolytic Virother. 2021, 10, 1–27. [Google Scholar] [CrossRef]
- Haddad, A.F.; Young, J.S.; Mummaneni, N.V.; Kasahara, N.; Aghi, M.K. Immunologic aspects of viral therapy for glioblastoma and implications for interactions with immunotherapies. J. Neurooncol. 2021, 152, 1–13. [Google Scholar] [CrossRef]
- van den Hengel, S.K.; Balvers, R.K.; Dautzenberg, I.J.; van den Wollenberg, D.J.; Kloezeman, J.J.; Lamfers, M.L.; Sillivis-Smit, P.A.; Hoeben, R.C. Heterogeneous reovirus susceptibility in human glioblastoma stem-like cell cultures. Cancer Gene Ther. 2013, 20, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Thomas, A.A.; Ernstoff, M.S.; Fadul, C.E. Immunotherapy for the treatment of glioblastoma. Cancer J. 2012, 18, 59–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Datsi, A.; Sorg, R.V. Dendritic Cell Vaccination of Glioblastoma: Road to Success or Dead End. Front. Immunol. 2021, 12, 770390. [Google Scholar] [CrossRef]
- Zeng, Y.; Chen, X. 90K predicts the prognosis of glioma patients and enhances tumor lysate-pulsed DC vaccine for immunotherapy of GBM in vitro. Aging 2021, 13, 8355–8368. [Google Scholar] [CrossRef]
- Baratta, M.G. Glioblastoma is “hot” for personalized vaccines. Nat. Rev. Cancer 2019, 19, 129. [Google Scholar] [CrossRef]
- Zhong, L.; Li, Y.; Xiong, L.; Wang, W.; Wu, M.; Yuan, T.; Yang, W.; Tian, C.; Miao, Z.; Wang, T.; et al. Small molecules in targeted cancer therapy: Advances, challenges, and future perspectives. Signal Transduct. Target. Ther. 2021, 6, 201. [Google Scholar] [CrossRef]
- Lee, Y.T.; Tan, Y.J.; Oon, C.E. Molecular targeted therapy: Treating cancer with specificity. Eur. J. Pharmacol. 2018, 834, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.P.; Adamson, D.C. Current FDA-Approved Therapies for High-Grade Malignant Gliomas. Biomedicines 2021, 9, 324. [Google Scholar] [CrossRef]
- Cohen, M.H.; Shen, Y.L.; Keegan, P.; Pazdur, R. FDA Drug Approval Summary: Bevacizumab (Avastin®) as Treatment of Recurrent Glioblastoma Multiforme. Oncologist 2009, 14, 1131–1138. [Google Scholar] [CrossRef]
- Brar, H.K.; Jose, J.; Wu, Z.; Sharma, M. Tyrosine Kinase Inhibitors for Glioblastoma Multiforme: Challenges and Opportunities for Drug Delivery. Pharmaceutics 2023, 15, 59. [Google Scholar] [CrossRef]
- Robinson, D.R.; Wu, Y.-M.; Lin, S.-F. The protein tyrosine kinase family of the human genome. Oncogene 2000, 19, 5548–5557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pottier, C.; Fresnais, M.; Gilon, M.; Jérusalem, G.; Longuespée, R.; Sounni, N.E. Tyrosine Kinase Inhibitors in Cancer: Breakthrough and Challenges of Targeted Therapy. Cancers 2020, 12, 731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Witt Hamer, P.C. Small molecule kinase inhibitors in glioblastoma: A systematic review of clinical studies. Neuro-Oncology 2010, 12, 304–316. [Google Scholar] [CrossRef]
- Aldaz, P.; Arozarena, I. Tyrosine Kinase Inhibitors in Adult Glioblastoma: An (Un)Closed Chapter? Cancers 2021, 13, 5799. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Ko, Y.T. Small molecule tyrosine kinase inhibitors in glioblastoma. Arch. Pharmacal Res. 2020, 43, 385–394. [Google Scholar] [CrossRef]
- Rich, J.N.; Reardon, D.A.; Peery, T.; Dowell, J.M.; Quinn, J.A.; Penne, K.L.; Wikstrand, C.J.; Duyn, L.B.V.; Dancey, J.E.; McLendon, R.E.; et al. Phase II Trial of Gefitinib in Recurrent Glioblastoma. J. Clin. Oncol. 2004, 22, 133–142. [Google Scholar] [CrossRef]
- Batchelor, T.T.; Duda, D.G.; di Tomaso, E.; Ancukiewicz, M.; Plotkin, S.R.; Gerstner, E.; Eichler, A.F.; Drappatz, J.; Hochberg, F.H.; Benner, T.; et al. Phase II study of cediranib, an oral pan-vascular endothelial growth factor receptor tyrosine kinase inhibitor, in patients with recurrent glioblastoma. J. Clin. Oncol. 2010, 28, 2817–2823. [Google Scholar] [CrossRef]
- Batchelor, T.T.; Mulholland, P.; Neyns, B.; Nabors, L.B.; Campone, M.; Wick, A.; Mason, W.; Mikkelsen, T.; Phuphanich, S.; Ashby, L.S.; et al. Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J. Clin. Oncol. 2013, 31, 3212–3218. [Google Scholar] [CrossRef] [Green Version]
- Lu-Emerson, C.; Duda, D.G.; Emblem, K.E.; Taylor, J.W.; Gerstner, E.R.; Loeffler, J.S.; Batchelor, T.T.; Jain, R.K. Lessons from anti-vascular endothelial growth factor and anti-vascular endothelial growth factor receptor trials in patients with glioblastoma. J. Clin. Oncol. 2015, 33, 1197–1213. [Google Scholar] [CrossRef]
- Graziani, G.; Szabó, C. Clinical perspectives of PARP inhibitors. Pharmacol. Res. 2005, 52, 109–118. [Google Scholar] [CrossRef]
- Yi, M.; Dong, B.; Qin, S.; Chu, Q.; Wu, K.; Luo, S. Advances and perspectives of PARP inhibitors. Exp. Hematol. Oncol. 2019, 8, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sim, H.W.; Galanis, E.; Khasraw, M. PARP Inhibitors in Glioma: A Review of Therapeutic Opportunities. Cancers 2022, 14, 1003. [Google Scholar] [CrossRef] [PubMed]
- Bisht, P.; Kumar, V.U.; Pandey, R.; Velayutham, R.; Kumar, N. Role of PARP Inhibitors in Glioblastoma and Perceiving Challenges as Well as Strategies for Successful Clinical Development. Front. Pharmacol. 2022, 13, 939570. [Google Scholar] [CrossRef]
- Echavidre, W.; Picco, V.; Faraggi, M.; Montemagno, C. Integrin-αvβ3 as a Therapeutic Target in Glioblastoma: Back to the Future? Pharmaceutics 2022, 14, 1053. [Google Scholar] [CrossRef]
- Rivera-Caraballo, K.A.; Nair, M.; Lee, T.J.; Kaur, B.; Yoo, J.Y. The complex relationship between integrins and oncolytic herpes Simplex Virus 1 in high-grade glioma therapeutics. Mol. Ther. Oncolytics 2022, 26, 63–75. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Hegi, M.E.; Gorlia, T.; Erridge, S.C.; Perry, J.; Hong, Y.-K.; Aldape, K.D.; Lhermitte, B.; Pietsch, T.; Grujicic, D.; et al. Cilengitide combined with standard treatment for patients with newly diagnosed glioblastoma with methylated MGMT promoter (CENTRIC EORTC 26071–22072 study): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2014, 15, 1100–1108. [Google Scholar] [CrossRef] [Green Version]
- Bell-McGuinn, K.M.; Matthews, C.M.; Ho, S.N.; Barve, M.; Gilbert, L.; Penson, R.T.; Lengyel, E.; Palaparthy, R.; Gilder, K.; Vassos, A.; et al. A phase II, single-arm study of the anti-α5β1 integrin antibody volociximab as monotherapy in patients with platinum-resistant advanced epithelial ovarian or primary peritoneal cancer. Gynecol. Oncol. 2011, 121, 273–279. [Google Scholar] [CrossRef] [Green Version]
- Aparicio-Blanco, J.; Sanz-Arriazu, L.; Lorenzoni, R.; Blanco-Prieto, M.J. Glioblastoma chemotherapeutic agents used in the clinical setting and in clinical trials: Nanomedicine approaches to improve their efficacy. Int. J. Pharm. 2020, 581, 119283. [Google Scholar] [CrossRef]
- Mecca, C.; Giambanco, I.; Donato, R.; Arcuri, C. Targeting mTOR in Glioblastoma: Rationale and Preclinical/Clinical Evidence. Dis. Markers 2018, 2018, 9230479. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.-F.; Wang, J.; Shao, W.; Wu, C.-P.; Chen, Z.-P.; To, S.-S.T.; Li, W.-P. Recent advances in the use of PI3K inhibitors for glioblastoma multiforme: Current preclinical and clinical development. Mol. Cancer 2017, 16, 100. [Google Scholar] [CrossRef] [Green Version]
- D’Alessandris, Q.G.; Martini, M.; Cenci, T.; Di Bonaventura, R.; Lauretti, L.; Stumpo, V.; Olivi, A.; Larocca, L.M.; Pallini, R.; Montano, N. Tailored therapy for recurrent glioblastoma. Report of a personalized molecular approach. J. Neurosurg. Sci. 2020, 67, 103–107. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Mukherjee, P. Targeting energy metabolism in brain cancer: Review and hypothesis. Nutr. Metab. 2005, 2, 30. [Google Scholar] [CrossRef] [Green Version]
- Rieger, J.; Bähr, O.; Maurer, G.D.; Hattingen, E.; Franz, K.; Brucker, D.; Walenta, S.; Kämmerer, U.; Coy, J.F.; Weller, M.; et al. ERGO: A pilot study of ketogenic diet in recurrent glioblastoma. Int. J. Oncol. 2014, 44, 1843–1852. [Google Scholar] [CrossRef] [Green Version]
- van der Louw, E.; Olieman, J.F.; van den Bemt, P.; Bromberg, J.E.C.; Oomen-de Hoop, E.; Neuteboom, R.F.; Catsman-Berrevoets, C.E.; Vincent, A. Ketogenic diet treatment as adjuvant to standard treatment of glioblastoma multiforme: A feasibility and safety study. Ther. Adv. Med. Oncol. 2019, 11, 1758835919853958. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, M.G.; Fenton, K.E.; Preul, M.C.; Rho, J.M.; Lynch, A.; Stafford, P.; Scheck, A.C. The ketogenic diet is an effective adjuvant to radiation therapy for the treatment of malignant glioma. PLoS ONE 2012, 7, e36197. [Google Scholar] [CrossRef] [PubMed]
- Woolf, E.C.; Scheck, A.C. The ketogenic diet for the treatment of malignant glioma. J. Lipid Res. 2015, 56, 5–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montella, L.; Sarno, F.; Altucci, L.; Cioffi, V.; Sigona, L.; Di Colandrea, S.; De Simone, S.; Marinelli, A.; Facchini, B.A.; De Vita, F.; et al. A Root in Synapsis and the Other One in the Gut Microbiome-Brain Axis: Are the Two Poles of Ketogenic Diet Enough to Challenge Glioblastoma? Front. Nutr. 2021, 8, 703392. [Google Scholar] [CrossRef] [PubMed]
- Klement, R.J.; Champ, C.E. Calories, carbohydrates, and cancer therapy with radiation: Exploiting the five R’s through dietary manipulation. Cancer Metastasis Rev. 2014, 33, 217–229. [Google Scholar] [CrossRef] [Green Version]
- U.S. National Library of Medicine. 16 Studies Found for: Ketogenic Diet—Glioblastoma Multiforme. Available online: https://clinicaltrials.gov/ct2/results?cond=Glioblastoma+Multiforme&term=ketogenic+diet&cntry=&state=&city=&dist= (accessed on 24 March 2023).
- Liao, W.; Fan, S.; Zheng, Y.; Liao, S.; Xiong, Y.; Li, Y.; Liu, J. Recent Advances on Glioblastoma Multiforme and Nano-drug Carriers: A Review. Curr. Med. Chem. 2019, 26, 5862–5874. [Google Scholar] [CrossRef]
- Filipczak, N.; Pan, J.; Yalamarty, S.S.K.; Torchilin, V.P. Recent advancements in liposome technology. Adv. Drug Deliv. Rev. 2020, 156, 4–22. [Google Scholar] [CrossRef]
- Chen, C.; Duan, Z.; Yuan, Y.; Li, R.; Pang, L.; Liang, J.; Xu, X.; Wang, J. Peptide-22 and Cyclic RGD Functionalized Liposomes for Glioma Targeting Drug Delivery Overcoming BBB and BBTB. ACS Appl. Mater. Interfaces 2017, 9, 5864–5873. [Google Scholar] [CrossRef]
- Liu, C.; Liu, X.N.; Wang, G.L.; Hei, Y.; Meng, S.; Yang, L.F.; Yuan, L.; Xie, Y. A dual-mediated liposomal drug delivery system targeting the brain: Rational construction, integrity evaluation across the blood-brain barrier, and the transporting mechanism to glioma cells. Int. J. Nanomed. 2017, 12, 2407–2425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Z.; Zhang, J.; Jiang, J.; He, Y.; Zhang, W.; Mo, X.; Kang, X.; Xu, Q.; Wang, B.; Huang, Y. Remodeling tumor immune microenvironment (TIME) for glioma therapy using multi-targeting liposomal codelivery. J. Immunother. Cancer 2020, 8, e000207. [Google Scholar] [CrossRef]
- Prencipe, F.; Diaferia, C.; Rossi, F.; Ronga, L.; Tesauro, D. Forward Precision Medicine: Micelles for Active Targeting Driven by Peptides. Molecules 2021, 26, 4049. [Google Scholar] [CrossRef] [PubMed]
- Doerflinger, A.; Quang, N.N.; Gravel, E.; Ducongé, F.; Doris, E. Aptamer-decorated polydiacetylene micelles with improved targeting of cancer cells. Int. J. Pharm. 2019, 565, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Quader, S.; Liu, X.; Chen, Y.; Mi, P.; Chida, T.; Ishii, T.; Miura, Y.; Nishiyama, N.; Cabral, H.; Kataoka, K. cRGD peptide-installed epirubicin-loaded polymeric micelles for effective targeted therapy against brain tumors. J. Control Release 2017, 258, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dai, S.; Wen, L.; Zhu, Y.; Tan, Y.; Qiu, G.; Meng, T.; Yu, F.; Yuan, H.; Hu, F. Enhancing Drug Delivery for Overcoming Angiogenesis and Improving the Phototherapy Efficacy of Glioblastoma by ICG-Loaded Glycolipid-Like Micelles. Int. J. Nanomed. 2020, 15, 2717–2732. [Google Scholar] [CrossRef] [Green Version]
- Du, K.; Xia, Q.; Sun, J.; Feng, F. Visible Light and Glutathione Dually Responsive Delivery of a Polymer-Conjugated Temozolomide Intermediate for Glioblastoma Chemotherapy. ACS Appl. Mater. Interfaces 2021, 13, 55851–55861. [Google Scholar] [CrossRef]
- Lotocki, V.; Yazdani, H.; Zhang, Q.; Gran, E.R.; Nyrko, A.; Maysinger, D.; Kakkar, A. Miktoarm Star Polymers with Environment-Selective ROS/GSH Responsive Locations: From Modular Synthesis to Tuned Drug Release through Micellar Partial Corona Shedding and/or Core Disassembly. Macromol. Biosci. 2021, 21, e2000305. [Google Scholar] [CrossRef]
- Jiao, X.; Yu, Y.; Meng, J.; He, M.; Zhang, C.J.; Geng, W.; Ding, B.; Wang, Z.; Ding, X. Dual-targeting and microenvironment-responsive micelles as a gene delivery system to improve the sensitivity of glioma to radiotherapy. Acta Pharm. Sin. B 2019, 9, 381–396. [Google Scholar] [CrossRef]
- Quader, S.; Liu, X.; Toh, K.; Su, Y.L.; Maity, A.R.; Tao, A.; Paraiso, W.K.D.; Mochida, Y.; Kinoh, H.; Cabral, H.; et al. Supramolecularly enabled pH- triggered drug action at tumor microenvironment potentiates nanomedicine efficacy against glioblastoma. Biomaterials 2021, 267, 120463. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhang, C.Y.; Wu, J.; Zhou, H.; Bai, R.; Shen, Z.; Deng, F.; Liu, Y.; Liu, J. PEG-Detachable Polymeric Micelles Self-Assembled from Amphiphilic Copolymers for Tumor-Acidity-Triggered Drug Delivery and Controlled Release. ACS Appl. Mater. Interfaces 2019, 11, 5701–5713. [Google Scholar] [CrossRef]
- Luo, Z.; Jin, K.; Pang, Q.; Shen, S.; Yan, Z.; Jiang, T.; Zhu, X.; Yu, L.; Pang, Z.; Jiang, X. On-Demand Drug Release from Dual-Targeting Small Nanoparticles Triggered by High-Intensity Focused Ultrasound Enhanced Glioblastoma-Targeting Therapy. ACS Appl. Mater. Interfaces 2017, 9, 31612–31625. [Google Scholar] [CrossRef] [PubMed]
- Do, H.T.; Bruelle, C.; Pham, D.D.; Jauhiainen, M.; Eriksson, O.; Korhonen, L.T.; Lindholm, D. Nerve growth factor (NGF) and pro-NGF increase low-density lipoprotein (LDL) receptors in neuronal cells partly by different mechanisms: Role of LDL in neurite outgrowth. J. Neurochem. 2016, 136, 306–315. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Li, D.; Zhao, J.; Song, J.; Zhao, Y. The role of the low-density lipoprotein receptor-related protein 1 (LRP-1) in regulating blood-brain barrier integrity. Rev. Neurosci. 2016, 27, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Sonoda, H.; Morimoto, H.; Yoden, E.; Koshimura, Y.; Kinoshita, M.; Golovina, G.; Takagi, H.; Yamamoto, R.; Minami, K.; Mizoguchi, A.; et al. A Blood-Brain-Barrier-Penetrating Anti-human Transferrin Receptor Antibody Fusion Protein for Neuronopathic Mucopolysaccharidosis II. Mol. Ther. 2018, 26, 1366–1374. [Google Scholar] [CrossRef] [PubMed]
- Somani, S.; Robb, G.; Pickard, B.S.; Dufès, C. Enhanced gene expression in the brain following intravenous administration of lactoferrin-bearing polypropylenimine dendriplex. J. Control Release 2015, 217, 235–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boado, R.J.; Pardridge, W.M. Brain and Organ Uptake in the Rhesus Monkey in Vivo of Recombinant Iduronidase Compared to an Insulin Receptor Antibody-Iduronidase Fusion Protein. Mol. Pharm. 2017, 14, 1271–1277. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Xin, H.; Ren, Q.; Gu, J.; Zhu, L.; Du, F.; Feng, C.; Xie, Y.; Sha, X.; Fang, X. Nanoparticles of 2-deoxy-D-glucose functionalized poly(ethylene glycol)-co-poly(trimethylene carbonate) for dual-targeted drug delivery in glioma treatment. Biomaterials 2014, 35, 518–529. [Google Scholar] [CrossRef]
- Wei, Y.; Sun, Y.; Wei, J.; Qiu, X.; Meng, F.; Storm, G.; Zhong, Z. Selective transferrin coating as a facile strategy to fabricate BBB-permeable and targeted vesicles for potent RNAi therapy of brain metastatic breast cancer in vivo. J. Control Release 2021, 337, 521–529. [Google Scholar] [CrossRef]
- Wankhede, M.; Bouras, A.; Kaluzova, M.; Hadjipanayis, C.G. Magnetic nanoparticles: An emerging technology for malignant brain tumor imaging and therapy. Expert Rev. Clin. Pharmacol. 2012, 5, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, K.; Bouras, A.; Bozec, D.; Ivkov, R.; Hadjipanayis, C. Magnetic hyperthermia therapy for the treatment of glioblastoma: A review of the therapy’s history, efficacy and application in humans. Int. J. Hyperth. 2018, 34, 1316–1328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ge, Y.; Zhong, Y.; Ji, G.; Lu, Q.; Dai, X.; Guo, Z.; Zhang, P.; Peng, G.; Zhang, K.; Li, Y. Preparation and characterization of Fe3O4@Au-C225 composite targeted nanoparticles for MRI of human glioma. PLoS ONE 2018, 13, e0195703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Q.; Dai, X.; Zhang, P.; Tan, X.; Zhong, Y.; Yao, C.; Song, M.; Song, G.; Zhang, Z.; Peng, G.; et al. Fe3O4@Au composite magnetic nanoparticles modified with cetuximab for targeted magneto-photothermal therapy of glioma cells. Int. J. Nanomed. 2018, 13, 2491–2505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shevtsov, M.; Nikolaev, B.; Marchenko, Y.; Yakovleva, L.; Skvortsov, N.; Mazur, A.; Tolstoy, P.; Ryzhov, V.; Multhoff, G. Targeting experimental orthotopic glioblastoma with chitosan-based superparamagnetic iron oxide nanoparticles (CS-DX-SPIONs). Int. J. Nanomed. 2018, 13, 1471–1482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tapeinos, C.; Marino, A.; Battaglini, M.; Migliorin, S.; Brescia, R.; Scarpellini, A.; De Julián Fernández, C.; Prato, M.; Drago, F.; Ciofani, G. Stimuli-responsive lipid-based magnetic nanovectors increase apoptosis in glioblastoma cells through synergic intracellular hyperthermia and chemotherapy. Nanoscale 2018, 11, 72–88. [Google Scholar] [CrossRef] [Green Version]
- Shirvalilou, S.; Khoei, S.; Khoee, S.; Raoufi, N.J.; Karimi, M.R.; Shakeri-Zadeh, A. Development of a magnetic nano-graphene oxide carrier for improved glioma-targeted drug delivery and imaging: In vitro and in vivo evaluations. Chem. Biol. Interact. 2018, 295, 97–108. [Google Scholar] [CrossRef]
- Xu, K.; Zhao, Z.; Zhang, J.; Xue, W.; Tong, H.; Liu, H.; Zhang, W. Albumin-stabilized manganese-based nanocomposites with sensitive tumor microenvironment responsivity and their application for efficient SiRNA delivery in brain tumors. J. Mater. Chem. B 2020, 8, 1507–1515. [Google Scholar] [CrossRef]
- Bockamp, E.; Rosigkeit, S.; Siegl, D.; Schuppan, D. Nano-Enhanced Cancer Immunotherapy: Immunology Encounters Nanotechnology. Cells 2020, 9, 2102. [Google Scholar] [CrossRef]
- Huang, P.; Wang, X.; Liang, X.; Yang, J.; Zhang, C.; Kong, D.; Wang, W. Nano-, micro-, and macroscale drug delivery systems for cancer immunotherapy. Acta Biomater. 2019, 85, 1–26. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, C.; Li, A.; Jing, W.; Sun, P.; Huang, X.; Liu, Y.; Zhang, S.; Du, W.; Zhang, R.; et al. Immunostimulant hydrogel for the inhibition of malignant glioma relapse post-resection. Nat. Nanotechnol. 2021, 16, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Li, T.F.; Xu, Y.H.; Li, K.; Wang, C.; Liu, X.; Yue, Y.; Chen, Z.; Yuan, S.J.; Wen, Y.; Zhang, Q.; et al. Doxorubicin-polyglycerol-nanodiamond composites stimulate glioblastoma cell immunogenicity through activation of autophagy. Acta Biomater. 2019, 86, 381–394. [Google Scholar] [CrossRef]
- Li, T.F.; Li, K.; Zhang, Q.; Wang, C.; Yue, Y.; Chen, Z.; Yuan, S.J.; Liu, X.; Wen, Y.; Han, M.; et al. Dendritic cell-mediated delivery of doxorubicin-polyglycerol-nanodiamond composites elicits enhanced anti-cancer immune response in glioblastoma. Biomaterials 2018, 181, 35–52. [Google Scholar] [CrossRef]
- Fana, M.; Gallien, J.; Srinageshwar, B.; Dunbar, G.L.; Rossignol, J. PAMAM Dendrimer Nanomolecules Utilized as Drug Delivery Systems for Potential Treatment of Glioblastoma: A Systematic Review. Int. J. Nanomed. 2020, 15, 2789–2808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sowińska, M.; Szeliga, M.; Morawiak, M.; Zabłocka, B.; Urbanczyk-Lipkowska, Z. Design, Synthesis and Activity of New N(1)-Alkyl Tryptophan Functionalized Dendrimeric Peptides against Glioblastoma. Biomolecules 2022, 12, 1116. [Google Scholar] [CrossRef]
- Gajbhiye, V.; Jain, N.K. The treatment of Glioblastoma Xenografts by surfactant conjugated dendritic nanoconjugates. Biomaterials 2011, 32, 6213–6225. [Google Scholar] [CrossRef]
- Janiszewska, J.; Posadas, I.; Játiva, P.; Bugaj-Zarebska, M.; Urbanczyk-Lipkowska, Z.; Ceña, V. Second Generation Amphiphilic Poly-Lysine Dendrons Inhibit Glioblastoma Cell Proliferation without Toxicity for Neurons or Astrocytes. PLoS ONE 2016, 11, e0165704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahoo, R.K.; Gupta, T.; Batheja, S.; Goyal, A.K.; Gupta, U. Surface Engineered Dendrimers: A Potential Nanocarrier for the Effective Management of Glioblastoma Multiforme. Curr. Drug Metab. 2022, 23, 708–722. [Google Scholar]
- Smith-Cohn, M.A.; Celiku, O.; Gilbert, M.R. Molecularly Targeted Clinical Trials. Neurosurg. Clin. N. Am. 2021, 32, 191–210. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. Available online: https://clinicaltrials.gov/ct2/results?cond=Glioblastoma+Multiforme&term=vehicle&cntry=&state=&city=&dist=&Search=Search&flds=abfkrv&type=Intr&phase=4&phase=0&phase=1&phase=2&phase=3; https://clinicaltrials.gov/ct2/results?term=liposomal&type=Intr&cond=Glioblastoma+Multiforme&phase=01234; https://clinicaltrials.gov/ct2/results?term=nanoparticles&type=Intr&cond=Glioblastoma+Multiforme&phase=01234; (accessed on 20 March 2023).
- Liang, S.F.; Zuo, F.F.; Yin, B.C.; Ye, B.C. Delivery of siRNA based on engineered exosomes for glioblastoma therapy by targeting STAT3. Biomater. Sci. 2022, 10, 1582–1590. [Google Scholar] [CrossRef]
- Tan, A.C.; Ashley, D.M.; López, G.Y.; Malinzak, M.; Friedman, H.S.; Khasraw, M. Management of glioblastoma: State of the art and future directions. CA Cancer J. Clin. 2020, 70, 299–312. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 2008, 455, 1061–1068. [Google Scholar] [CrossRef] [Green Version]
- Chryplewicz, A.; Scotton, J.; Tichet, M.; Zomer, A.; Shchors, K.; Joyce, J.A.; Homicsko, K.; Hanahan, D. Cancer cell autophagy, reprogrammed macrophages, and remodeled vasculature in glioblastoma triggers tumor immunity. Cancer Cell 2022, 40, 1111–1127.e9. [Google Scholar] [CrossRef] [PubMed]
- Spinazzi, E.F.; Argenziano, M.G.; Upadhyayula, P.S.; Banu, M.A.; Neira, J.A.; Higgins, D.M.O.; Wu, P.B.; Pereira, B.; Mahajan, A.; Humala, N.; et al. Chronic convection-enhanced delivery of topotecan for patients with recurrent glioblastoma: A first-in-patient, single-centre, single-arm, phase 1b trial. Lancet Oncol. 2022, 23, 1409–1418. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, D.; Zhang, H.; Hu, G.; Guo, B. Recent development of contrast agents for magnetic resonance and multimodal imaging of glioblastoma. J. Nanobiotechnol. 2022, 20, 284. [Google Scholar] [CrossRef] [PubMed]



| NCT Number | Title | Conditions | Outcome Measures | Phases | Last Update Posted |
|---|---|---|---|---|---|
| NCT05768919 | Study of Liposomal Curcumin in Combination With RT and TMZ in Patients With Newly Diagnosed High-Grade Gliomas | Glioblastoma | The number of observed Dose Limiting Toxicity (DLTs) The incidence of Adverse Events The proportion of patients at each dose level who receive at least 80% of the planned infusions of LC, 80% of RT, and 60% of TMZ during the first 10 weeks of treatment. Overall Survival (OS) Progression-free survival (PFS) | Phase 1 Phase 2 | 15 March 2023 |
| NCT04573140 | A Study of RNA-lipid Particle (RNA-LP) Vaccines for Newly Diagnosed Pediatric High-Grade Gliomas (pHGG) and Adult Glioblastoma (GBM) | Adult Glioblastoma | Manufacturing feasibility Safety of RNA-LP vaccine Determination of Maximum Tolerated Dose | Phase 1 | 21 February 2023 |
| NCT01906385 | Maximum Tolerated Dose, Safety, and Efficacy of Rhenium Nanoliposomes in Recurrent Glioma (ReSPECT) | Glioma | Phase 1: Maximum Tolerated Dose Phase 2: Overall Survival Phase 1: Dose Distribution Phase 1: Response rate Phase 1: Survival Phase 1: Safety of single dose of treatment Phase 2: Safety and tolerability of 186RNL Phase 2: Objective response rate (ORR) Phase 2: Progression-free survival at 6 months (PFS-6) Phase 2: Progression-free survival (PFS) from the initiation of study to first documented progression Phase 2: Quality of Life | Phase 1 Phase 2 | 12 December 2022 |
| NCT05460507 | Safety & Efficacy/Tolerability of Rhenium-186 NanoLiposomes (186RNL) for Patients Who Received a Prior 186RNL Treatment | Glioma | Assessment of safety and tolerability of a second dose of 186RNL by CED as part of standard of care > 30 days following first dose. Overall Survival Dose Distribution Overall Response Rate Progression-free survival | Phase 1 | 23 December 2022 |
| NCT04881032 | AGuIX Nanoparticles With Radiotherapy Plus Concomitant Temozolomide in the Treatment of Newly Diagnosed Glioblastoma | Glioblastoma | The recommended dose (phase I) of AGuIX in combination with TMZ and radiotherapy during the radio-chemotherapy period 6-month Progression-Free Survival (PFS) rate (phase II) Pharmacokinetic Cmax of AGuIX Pharmacokinetic Tmax of AGuIX Pharmacokinetic AUC of AGuIX Pharmacokinetic t1/2 of AGuIX distribution of AGuIX Overall Survival Progression-Free Survival (PFS) Toxicity (CTCAE criteria) | Phase 1 Phase 2 | 8 March 2023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yalamarty, S.S.K.; Filipczak, N.; Li, X.; Subhan, M.A.; Parveen, F.; Ataide, J.A.; Rajmalani, B.A.; Torchilin, V.P. Mechanisms of Resistance and Current Treatment Options for Glioblastoma Multiforme (GBM). Cancers 2023, 15, 2116. https://doi.org/10.3390/cancers15072116
Yalamarty SSK, Filipczak N, Li X, Subhan MA, Parveen F, Ataide JA, Rajmalani BA, Torchilin VP. Mechanisms of Resistance and Current Treatment Options for Glioblastoma Multiforme (GBM). Cancers. 2023; 15(7):2116. https://doi.org/10.3390/cancers15072116
Chicago/Turabian StyleYalamarty, Satya Siva Kishan, Nina Filipczak, Xiang Li, Md Abdus Subhan, Farzana Parveen, Janaína Artem Ataide, Bharat Ashok Rajmalani, and Vladimir P. Torchilin. 2023. "Mechanisms of Resistance and Current Treatment Options for Glioblastoma Multiforme (GBM)" Cancers 15, no. 7: 2116. https://doi.org/10.3390/cancers15072116