Advances in Intralesional Therapy for Locoregionally Advanced and Metastatic Melanoma: Five Years of Progress
Abstract
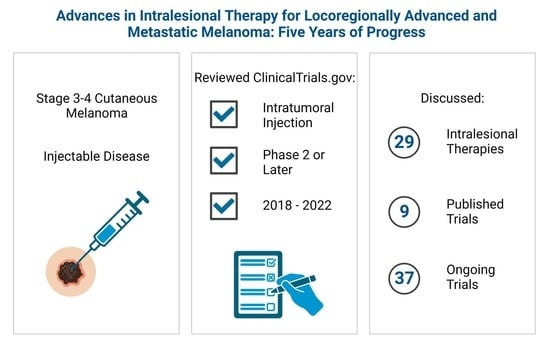
:Simple Summary
Abstract
1. Introduction
2. Xanthene Dyes
2.1. PV-10
2.2. Cytokines
2.2.1. Interleukin-2 (IL-2, Aldesleukin)
2.2.2. Tavokinogene Telseplasmid (Tavo)
2.3. Antibody–Cytokine Fusion Proteins
2.3.1. Darleukin (L19IL2)
2.3.2. Daromun (L19IL2 and L19TNF)
2.4. Oncolytic Viral Therapies
2.4.1. Talimogene Laherparepvec
2.4.2. RP1 (Vusolimogene Oderparepvec)
2.4.3. OrienX010
2.4.4. ONCOS-102 (Ad5/3-Δ24-GM-CSF)
2.4.5. BT-001
2.4.6. Canerpaturev (C-REV, TBI-1401, HF10)
2.4.7. Coxsackievirus A21 (CVA21, CAVATAK, V937, Gebasaxturev)
2.4.8. PVSRIPO (Lerapolturev)
2.4.9. OBP-301 (Telomelysin)
2.4.10. Voyager-V1 (VV1, VSV-IFNβ-NIS)
2.4.11. Ad-p53
2.4.12. RheoSwitch Therapeutic System
2.5. Toll-like Receptor 9 (TLR9) Agonists
2.5.1. Tilsotolimod (IMO-2125)
2.5.2. SD-101
2.5.3. Vidutolimod (CMP-001)
2.5.4. Cavrotolimod (AST-008)
2.6. Toll-like Receptor 7/8 (TLR 7/8) Agonists
NKTR-262
2.7. Immune Checkpoint Inhibitors
2.8. Other Therapeutics
2.8.1. LL37
2.8.2. Hiltonol (Polyinosinic-Polycytidylic Acid-Poly-I-Lysine Carboxymethylcellulose, Poly-ICLC)
2.8.3. APX005M
2.8.4. Dendritic Cell Therapy
2.8.5. INT230-6
2.8.6. Polidocanol
3. Conclusions
Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Thompson, J.F.; Mozzillo, N.; Ross, M.I. Local Melanoma Recurrence, Satellitosis, and In-transit Metastasis: Incidence, Outcomes, and Selection of Treatment Options. In Cutaneous Melanoma; Balch, C.M., Atkins, M.B., Garbe, C., Gershenwald, J.E., Halpern, A.C., Kirkwood, J.M., McArthur, G.A., Thompson, J.F., Sober, A.J., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 867–894. [Google Scholar] [CrossRef]
- Hodi, F.S.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Wagstaff, J.; Dummer, R. Nivolumab plus ipilimumab or nivolumab alone versus ipilimumab alone in advanced melanoma (CheckMate 067): 4-year outcomes of a multicentre, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1480–1492. [Google Scholar] [CrossRef] [PubMed]
- Long, G.V.; Schachter, J.; Ribas, A.; Arance, A.M.; Grob, J.-J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.M.; Lotem, M. 4-year survival and outcomes after cessation of pembrolizumab (pembro) after 2-years in patients (pts) with ipilimumab (ipi)-naive advanced melanoma in KEYNOTE-006. Am. Soc. Clin. Oncol. 2018, 36 (Suppl. 15), 9503. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef] [PubMed]
- Morton, D.L.; Eilber, F.R.; Holmes, E.C.; Hunt, J.S.; Ketcham, A.S.; Silverstein, M.J.; Sparks, F.C. BCG immunotherapy of malignant melanoma: Summary of a seven-year experience. Ann. Surg. 1974, 180, 635. [Google Scholar] [CrossRef]
- Bast, R.C., Jr.; Zbar, B.; Borsos, T.; Rapp, H.J. BCG and cancer. N. Engl. J. Med. 1974, 290, 1458–1469. [Google Scholar] [CrossRef]
- Agarwala, S.S.; Neuberg, D.; Park, Y.; Kirkwood, J.M. Mature results of a phase III randomized trial of bacillus Calmette–Guerin (BCG) versus observation and BCG plus dacarbazine versus BCG in the adjuvant therapy of American Joint Committee on Cancer Stage I–III melanoma (E1673) A trial of the Eastern Cooperative Oncology Group. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2004, 100, 1692–1698. [Google Scholar]
- Robinson, J.C. Risks of BCG intralesional therapy: An experience with melanoma. J. Surg. Oncol. 1977, 9, 587–593. [Google Scholar] [CrossRef]
- Ito, A.; Watanabe, H.; Naito, M.; Aoyama, H.; Nakagawa, Y.; Fujimoto, N. Induction of thyroid tumors in (C57BL/6N × C3H/N) F1 mice by oral administration of 9-3′, 4′, 5′, 6′-tetrachloro-o-carboxy phenyl-6-hydroxy-2, 4, 5, 7-tetraiodo-3-isoxanthone sodium (food red 105, rose bengal B). J. Natl. Cancer Inst. 1986, 77, 277–281. [Google Scholar]
- Wachter, E.A.; Dees, C.; Harkins, J.; Fisher, W.G.; Scott, T. Functional imaging photosensitizers using multiphoton microscopy. Multiphoton Microsc. Biomed. Sci. II Int. Soc. Opt. Photonics 2002, 143–147. [Google Scholar]
- Mousavi, H.; Zhang, X.; Gillespie, S.; Wachter, E.; Hersey, P. Rose Bengal induces dual modes of cell death in melanoma cells and has clinical activity against melanoma. Melanoma Res. 2006, 16, S8. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Innamarato, P.P.; Kodumudi, K.; Weber, A.; Nemoto, S.; Robinson, J.L.; Crago, G.; McCardle, T.; Royster, E.; Sarnaik, A.A.; et al. Intralesional rose bengal in melanoma elicits tumor immunity via activation of dendritic cells by the release of high mobility group box 1. Oncotarget 2016, 7, 37893–37905. [Google Scholar] [CrossRef] [PubMed]
- Dumitriu, I.E.; Baruah, P.; Bianchi, M.E.; Manfredi, A.A.; Rovere-Querini, P. Requirement of HMGB1 and RAGE for the maturation of human plasmacytoid dendritic cells. Eur. J. Immunol. 2005, 35, 2184–2190. [Google Scholar] [CrossRef]
- Tian, J.; Avalos, A.M.; Mao, S.-Y.; Chen, B.; Senthil, K.; Wu, H.; Parroche, P.; Drabic, S.; Golenbock, D.; Sirois, C. Toll-like receptor 9–dependent activation by DNA-containing immune complexes is mediated by HMGB1 and RAGE. Nat. Immunol. 2007, 8, 487–496. [Google Scholar] [CrossRef]
- Apetoh, L.; Ghiringhelli, F.; Tesniere, A.; Obeid, M.; Ortiz, C.; Criollo, A.; Mignot, G.; Maiuri, M.C.; Ullrich, E.; Saulnier, P. Toll-like receptor 4–dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat. Med. 2007, 13, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Gershenwald, J.E.; Scolyer, R.A.; Hess, K.R.; Sondak, V.K.; Long, G.V.; Ross, M.I.; Lazar, A.J.; Faries, M.B.; Kirkwood, J.M.; McArthur, G.A.; et al. Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 472–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, J.F.; Agarwala, S.S.; Smithers, B.M.; Ross, M.I.; Scoggins, C.R.; Coventry, B.J.; Neuhaus, S.J.; Minor, D.R.; Singer, J.M.; Wachter, E.A. Phase 2 Study of Intralesional PV-10 in Refractory Metastatic Melanoma. Ann. Surg. Oncol. 2015, 22, 2135–2142. [Google Scholar] [CrossRef] [Green Version]
- Lippey, J.; Bousounis, R.; Behrenbruch, C.; McKay, B.; Spillane, J.; Henderson, M.A.; Speakman, D.; Gyorki, D.E. Intralesional PV-10 for in-transit melanoma-A single-center experience. J. Surg. Oncol. 2016, 114, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Read, T.A.; Smith, A.; Thomas, J.; David, M.; Foote, M.; Wagels, M.; Barbour, A.; Smithers, B.M. Intralesional PV-10 for the treatment of in-transit melanoma metastases-Results of a prospective, non-randomized, single center study. J. Surg. Oncol. 2018, 117, 579–587. [Google Scholar] [CrossRef] [Green Version]
- Foote, M.; Read, T.; Thomas, J.; Wagels, M.; Burmeister, B.; Smithers, B.M. Results of a phase II, open-label, non-comparative study of intralesional PV-10 followed by radiotherapy for the treatment of in-transit or metastatic melanoma. J. Surg. Oncol. 2017, 115, 891–897. [Google Scholar] [CrossRef] [Green Version]
- Agarwala, S.S.; Ross, M.I.; Zager, J.S.; Shirai, K.; Essner, R.; Smithers, B.M.; Atkinson, V.; Wachter, E.A. Phase 1b study of PV-10 and anti-PD-1 in advanced cutaneous melanoma. J. Clin. Oncol. 2019, 37, 9559. [Google Scholar] [CrossRef]
- Zager, J.S.; Sarnaik, A.A.; Pilon-Thomas, S.; Beatty, M.; Han, D.; Lu, G.; Agrawala, S.S.; Ross, M.I.; Shirai, K.; Essner, R.; et al. Response for combination of PV-10 autolytic immunotherapy and immune checkpoint blockade in checkpoint-refractory patients. In Proceedings of the Oral Presentation at Melanoma Bridge, Virtual, 3–5 December 2020. [Google Scholar]
- Fernandez-Penas, P.; Carlino, M.S.; Tsai, K.K.; Atkinson, V.G.; Shaheen, M.; Thomas, S.; Mihalcioiu, C.; Van Hagen, T.; Roberts-Thompson, R.; Haydon, A.; et al. Durable responses and immune activation with intratumoral electroportation of pIL-12 plus pembrolizumab in actively progressing anti-PD-1 refractory advanced melanoma: KEYNOTE 695 interim data. In Proceedings of the Society for Immunotherapy of Cancer Annual Meeting, Virtual, 9–14 November 2020. [Google Scholar]
- Gastman, B.; Robert, C.; Gogas, H.; Rutkowski, P.; Long, G.V.; Chaney, M.F.; Joshi, H.; Lin, Y.-L.; Snyder, W.; Chesney, J.A. Primary analysis of a phase 2, open-label, multicenter trial of talimogene laherparepvec (T-VEC) plus pembrolizumab (pembro) for the treatment (Tx) of patients (pts) with advanced melanoma (MEL) who progressed on prior anti–PD-1 therapy: MASTERKEY-115. J. Clin. Oncol. 2022, 40, 9518. [Google Scholar] [CrossRef]
- Replimune Provides New Clinical Data, Broad Program Update and Future Development Strategy for Its Tumor-Directed Oncolytic Immunotherapies; Replimune: Woburn, MA, USA, 2022.
- Replimune Announces Positive Initial Data from the Anti-PD1 Failed Melanoma Cohort of the IGNYTE Clinical Trial & an RP2/3 Program Update; Replimune: Woburn, MA, USA, 2022.
- Nemunaitis, J.J.; Linette, G.P.; Hamid, O.; Agarwala, S.S.; Starodub, A.; Sun, L.; Lebel, F.; Barrett, J.A.; Lewis, J. Regulated intratumoral expression of IL-12 as a basis for combination therapy in melanoma. J. Transl. Med. 2014, 12, O11. [Google Scholar] [CrossRef] [Green Version]
- O’Day, S.; Perez, C.; Wise-Draper, T.; Hanna, G.; Bhatia, S.; Kelly, C.; Medina, T.; Laux, D.; Daud, A.; Chandra, S. 423 Safety and preliminary efficacy of intratumoral cavrotolimod (AST-008), a spherical nucleic acid TLR9 agonist, in combination with pembrolizumab in patients with advanced solid tumors. BMJ Spec. J. 2020, 8, A1–A559. [Google Scholar] [CrossRef]
- El-Khoeuiry, A.; Siu, L.; Azad, N.; Walters, I.; Bender, L.; Kamen, L.; Olszanski, A. Phase I/II evaluation of intratumoral INT230-6 for the treatment of solid tumors. Ann. Oncol. 2018, 29, viii413. [Google Scholar] [CrossRef]
- Wrangle, J.M.; Patterson, A.; Johnson, C.B.; Neitzke, D.J.; Mehrotra, S.; Denlinger, C.E.; Paulos, C.M.; Li, Z.; Cole, D.J.; Rubinstein, M.P. IL-2 and Beyond in Cancer Immunotherapy. J. Interferon Cytokine Res. 2018, 38, 45–68. [Google Scholar] [CrossRef] [PubMed]
- Atkins, M.B.; Lotze, M.T.; Dutcher, J.P.; Fisher, R.I.; Weiss, G.; Margolin, K.; Abrams, J.; Sznol, M.; Parkinson, D.; Hawkins, M. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: Analysis of 270 patients treated between 1985 and 1993. J. Clin. Oncol. 1999, 17, 2105. [Google Scholar] [CrossRef] [PubMed]
- Gutwald, J.G.J.; Groth, W.; Mahrle, G. Perilumoral injections of interleukin 2 induce tumour regression in metastatic malignant melanoma. Br. J. Dermatol. 1994, 130, 541–542. [Google Scholar] [CrossRef]
- Weide, B.; Derhovanessian, E.; Pflugfelder, A.; Eigentler, T.K.; Radny, P.; Zelba, H.; Pföhler, C.; Pawelec, G.; Garbe, C. High response rate after intratumoral treatment with interleukin-2. Cancer 2010, 116, 4139–4146. [Google Scholar] [CrossRef]
- Weide, B.; Martens, A.; Wistuba-Hamprecht, K.; Zelba, H.; Maier, L.; Lipp, H.-P.; Klumpp, B.D.; Soffel, D.; Eigentler, T.K.; Garbe, C. Combined treatment with ipilimumab and intratumoral interleukin-2 in pretreated patients with stage IV melanoma—safety and efficacy in a phase II study. Cancer Immunol. Immunother. 2017, 66, 441–449. [Google Scholar] [CrossRef]
- Trinchieri, G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003, 3, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, C.; Lesinski, G.B. Immunomodulatory cytokines as therapeutic agents for melanoma. Immunotherapy 2011, 3, 673–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinzerling, L.; Burg, G.; Dummer, R.; Maier, T.; Oberholzer, P.A.; Schultz, J.; Elzaouk, L.; Pavlovic, J.; Moelling, K. Intratumoral injection of DNA encoding human interleukin 12 into patients with metastatic melanoma: Clinical efficacy. Hum. Gene Ther. 2005, 16, 35–48. [Google Scholar] [CrossRef] [Green Version]
- Canton, D.A.; Shirley, S.; Wright, J.; Connolly, R.; Burkart, C.; Mukhopadhyay, A.; Twitty, C.; Qattan, K.E.; Campbell, J.S.; Le, M.H.; et al. Melanoma treatment with intratumoral electroporation of tavokinogene telseplasmid (pIL-12, tavokinogene telseplasmid). Immunotherapy 2017, 9, 1309–1321. [Google Scholar] [CrossRef] [Green Version]
- Heller, L.C.; Jaroszeski, M.J.; Coppola, D.; Heller, R. Comparison of electrically mediated and liposome-complexed plasmid DNA delivery to the skin. Genet. Vaccines 2008, 6, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curtsinger, J.M.; Mescher, M.F. Inflammatory cytokines as a third signal for T cell activation. Curr. Opin. Immunol. 2010, 22, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Algazi, A.; Bhatia, S.; Agarwala, S.; Molina, M.; Lewis, K.; Faries, M.; Fong, L.; Levine, L.P.; Franco, M.; Oglesby, A.; et al. Intratumoral delivery of tavokinogene telseplasmid yields systemic immune responses in metastatic melanoma patients. Ann. Oncol. 2020, 31, 532–540. [Google Scholar] [CrossRef] [Green Version]
- Schrama, D.; Reisfeld, R.A.; Becker, J.C. Antibody targeted drugs as cancer therapeutics. Nat. Rev. Drug Discov. 2006, 5, 147–159. [Google Scholar] [CrossRef]
- Neri, D.; Weide, B.; Eigentler, T.K.; Pflugfelder, A.; Zelba, H.; Pawelec, G.P.; Giovannoni, L.; Ruffini, P.A.; Elia, G.; Gutzmer, R.; et al. Intralesional treatment of stage III metastatic melanoma patients with L19-IL2: Clinical and systemic immunological responses. J. Clin. Oncol. 2014, 32, 9041. [Google Scholar] [CrossRef]
- Halin, C.; Gafner, V.; Villani, M.E.; Borsi, L.; Berndt, A.; Kosmehl, H.; Zardi, L.; Neri, D. Synergistic therapeutic effects of a tumor targeting antibody fragment, fused to interleukin 12 and to tumor necrosis factor α. Cancer Res. 2003, 63, 3202–3210. [Google Scholar]
- Danielli, R.; Patuzzo, R.; Di Giacomo, A.M.; Gallino, G.; Maurichi, A.; Di Florio, A.; Cutaia, O.; Lazzeri, A.; Fazio, C.; Miracco, C.; et al. Intralesional administration of L19-IL2/L19-TNF in stage III or stage IVM1a melanoma patients: Results of a phase II study. Cancer Immunol. Immunother. 2015, 64, 999–1009. [Google Scholar] [CrossRef]
- Miura, J.T.; Zager, J.S. Neo-DREAM study investigating Daromun for the treatment of clinical stage IIIB/C melanoma. Future Oncol. 2019, 15, 3665–3674. [Google Scholar] [CrossRef] [PubMed]
- Marelli, G.; Howells, A.; Lemoine, N.R.; Wang, Y. Oncolytic Viral Therapy and the Immune System: A Double-Edged Sword Against Cancer. Front. Immunol. 2018, 9, 866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuhara, H.; Ino, Y.; Todo, T. Oncolytic virus therapy: A new era of cancer treatment at dawn. Cancer Sci. 2016, 107, 1373–1379. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, H.L.; Ruby, C.E.; Hughes, T.; Slingluff, C.L. Current status of granulocyte–macrophage colony-stimulating factor in the immunotherapy of melanoma. J. Immunother. Cancer 2014, 2, 11. [Google Scholar] [CrossRef]
- Si, Z.; Hersey, P.; Coates, A. Clinical responses and lymphoid infiltrates in metastatic melanoma following treatment with intralesional GM-CSF. Melanoma Res. 1996, 6, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Nasi, M.L.; Lieberman, P.; Busam, K.J.; Prieto, V.; Panageas, K.S.; Lewis, J.J.; Houghton, A.N.; Chapman, P.B. Intradermal injection of granulocyte-macrophage colony-stimulating factor (GM-CSF) in patients with metastatic melanoma recruits dendritic cells. Cytokines Cell. Mol. 1999, 5, 139–144. [Google Scholar]
- Andtbacka, R.H.; Ross, M.; Puzanov, I.; Milhem, M.; Collichio, F.; Delman, K.A.; Amatruda, T.; Zager, J.S.; Cranmer, L.; Hsueh, E.; et al. Patterns of Clinical Response with Talimogene Laherparepvec (T-VEC) in Patients with Melanoma Treated in the OPTiM Phase III Clinical Trial. Ann. Surg. Oncol. 2016, 23, 4169–4177. [Google Scholar] [CrossRef] [Green Version]
- Andtbacka, R.H.; Kaufman, H.L.; Collichio, F.; Amatruda, T.; Senzer, N.; Chesney, J.; Delman, K.A.; Spitler, L.E.; Puzanov, I.; Agarwala, S.S.; et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J. Clin. Oncol. 2015, 33, 2780–2788. [Google Scholar] [CrossRef]
- Dummer, R.; Gyorki, D.E.; Hyngstrom, J.; Berger, A.C.; Conry, R.; Demidov, L.; Sharma, A.; Treichel, S.A.; Radcliffe, H.; Gorski, K.S.; et al. Neoadjuvant talimogene laherparepvec plus surgery versus surgery alone for resectable stage IIIB-IVM1a melanoma: A randomized, open-label, phase 2 trial. Nat. Med. 2021, 27, 1789–1796. [Google Scholar] [CrossRef]
- York, I.A.; Roop, C.; Andrews, D.W.; Riddell, S.R.; Graham, F.L.; Johnson, D.C. A cytosolic herpes simplex virus protein inhibits antigen presentation to CD8+ T lymphocytes. Cell 1994, 77, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Malvehy, J.; Samoylenko, I.; Schadendorf, D.; Gutzmer, R.; Grob, J.J.; Sacco, J.J.; Gorski, K.S.; Anderson, A.; Pickett, C.A.; Liu, K.; et al. Talimogene laherparepvec upregulates immune-cell populations in non-injected lesions: Findings from a phase II, multicenter, open-label study in patients with stage IIIB-IVM1c melanoma. J. Immunother. Cancer 2021, 9, e001621. [Google Scholar] [CrossRef] [PubMed]
- Chesney, J.; Puzanov, I.; Collichio, F.; Singh, P.; Milhem, M.M.; Glaspy, J.; Hamid, O.; Ross, M.; Friedlander, P.; Garbe, C.; et al. Randomized, Open-Label Phase II Study Evaluating the Efficacy and Safety of Talimogene Laherparepvec in Combination with Ipilimumab Versus Ipilimumab Alone in Patients with Advanced, Unresectable Melanoma. J. Clin. Oncol. 2018, 36, 1658–1667. [Google Scholar] [CrossRef] [PubMed]
- Chesney, J.A.; Ribas, A.; Long, G.V.; Kirkwood, J.M.; Dummer, R.; Puzanov, I.; Hoeller, C.; Gajewski, T.F.; Gutzmer, R.; Rutkowski, P.; et al. Randomized, Double-Blind, Placebo-Controlled, Global Phase III Trial of Talimogene Laherparepvec Combined with Pembrolizumab for Advanced Melanoma. J. Clin. Oncol. 2023, 41, 528–540. [Google Scholar] [CrossRef]
- Zager, J.S.; van Akkooi, A.C.J. Talimogene Laherparepvec in Combination with Immunotherapy, A Viable Option? Ann. Surg. Oncol. 2022, 30, 1279–1281. [Google Scholar] [CrossRef]
- Roulstone, V.; Kyula, J.; Thomas, S.; Kuncheria, L.; Bommareddy, P.K.; Smith, H.; Whittock, H.; Coffin, R.S.; Harrington, K. Immunomodulatory effects of a novel, enhanced potency gibbon ape leukaemia virus (GALV) fusogenic membrane glycoprotein-expressing herpes simplex virus platform with increased efficacy combined with anti PD-1 therapy. Cancer Res. 2021, 81, 1917. [Google Scholar] [CrossRef]
- Wei, D.; Xu, J.; Liu, X.-Y.; Chen, Z.-N.; Bian, H. Fighting cancer with viruses: Oncolytic virus therapy in China. Hum. Gene Ther. 2018, 29, 151–159. [Google Scholar] [CrossRef]
- Kuryk, L.; Møller, A.-S.W.; Jaderberg, M. Combination of immunogenic oncolytic adenovirus ONCOS-102 with anti-PD-1 pembrolizumab exhibits synergistic antitumor effect in humanized A2058 melanoma huNOG mouse model. Oncoimmunology 2019, 8, e1532763. [Google Scholar] [CrossRef]
- Semmrich, M.; Marchand, J.-B.; Fend, L.; Rehn, M.; Silvestre, N.; Mårtensson, L.; Foloppe, J.; Teige, I.; Quéméneur, E.; Frendeus, B. 594 BT-001, an oncolytic vaccinia virus armed with a Treg-depleting human recombinant anti-CTLA4 antibody and GM-CSF to target the tumor microenvironment. BMJ Spec. J. 2020. [Google Scholar] [CrossRef]
- Eissa, I.R.; Naoe, Y.; Bustos-Villalobos, I.; Ichinose, T.; Tanaka, M.; Zhiwen, W.; Mukoyama, N.; Morimoto, T.; Miyajima, N.; Hitoki, H.; et al. Genomic Signature of the Natural Oncolytic Herpes Simplex Virus HF10 and Its Therapeutic Role in Preclinical and Clinical Trials. Front. Oncol. 2017, 7, 149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andtbacka, R.H.; Ross, M.I.; Agarwala, S.S.; Taylor, M.H.; Vetto, J.T.; Neves, R.I.; Daud, A.; Khong, H.T.; Ungerleider, R.S.; Tanaka, M. Final results of a phase II multicenter trial of HF10, a replication-competent HSV-1 oncolytic virus, and ipilimumab combination treatment in patients with stage IIIB-IV unresectable or metastatic melanoma. J. Clin. Oncol. 2017, 35, 9510. [Google Scholar] [CrossRef]
- Yokota, K.; Isei, T.; Uhara, H.; Fujisawa, Y.; Takenouchi, T.; Kiyohara, Y.; Uchi, H.; Saruta, H.; Ihn, H.; Inozume, T.; et al. Final results from phase II combination with Canerpaturev (formerly HF10), an oncolytic viral immunotherapy, and ipilimumab in unresectable or metastatic melanoma in 2nd or later line treatment. In Proceedings of the European Society for Medical Oncology Congress, Barcelona, Spain, 27 September–1 October, 2019. [Google Scholar]
- Bradley, S.; Jakes, A.D.; Harrington, K.; Pandha, H.; Melcher, A.; Errington-Mais, F. Applications of coxsackievirus A21 in oncology. Oncolytic Virother. 2014, 3, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Andtbacka, R.H.I.; Curti, B.; Daniels, G.A.; Hallmeyer, S.; Whitman, E.D.; Lutzky, J.; Spitler, L.E.; Zhou, K.; Bommareddy, P.K.; Grose, M.; et al. Clinical Responses of Oncolytic Coxsackievirus A21 (V937) in Patients with Unresectable Melanoma. J. Clin. Oncol. 2021, 39, 3829–3838. [Google Scholar] [CrossRef]
- Walton, R.W.; Brown, M.C.; Sacco, M.T.; Gromeier, M. Engineered oncolytic poliovirus PVSRIPO subverts MDA5-dependent innate immune responses in cancer cells. J. Virol. 2018, 92, e00879-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawashima, T.; Kagawa, S.; Kobayashi, N.; Shirakiya, Y.; Umeoka, T.; Teraishi, F.; Taki, M.; Kyo, S.; Tanaka, N.; Fujiwara, T. Telomerase-specific replication-selective virotherapy for human cancer. Clin. Cancer Res. 2004, 10, 285–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powell, S.; Patel, M.R.; Merchan, J.R.; Cripe, T.P.; Strauss, J.; Diaz, R.M.; Packiriswamy, N.; Brunton, B.A.; Upreti, D.; Khan, R. Abstract CT051: Preliminary correlative and clinical data from a first-in-human (FIH) study of the intratumoral (IT) oncolytic virotherapy, Voyager-V1, in patients with solid tumors. Cancer Res. 2018, 78, CT051. [Google Scholar] [CrossRef]
- Lutzky, J.; Marron, T.U.; Powell, S.F.; Johnson, D.H.; Patel, M.; El-Khoueiry, A.B.; Sarantopoulos, J.; Dadi-Mehmetaj, S.; Russell, L.; Russell, S.J. Optimization of Voyager V1 (VV1) oncolytic virus systemic delivery in combination with cemiplimab and ipilimumab in patients with melanoma and non–small cell lung cancer (NSCLC). Am. Soc. Clin. Oncol. 2022, 40, TPS9595. [Google Scholar] [CrossRef]
- Zhang, W.W.; Li, L.; Li, D.; Liu, J.; Li, X.; Li, W.; Xu, X.; Zhang, M.J.; Chandler, L.A.; Lin, H.; et al. The First Approved Gene Therapy Product for Cancer Ad-p53 (Gendicine): 12 Years in the Clinic. Hum. Gene 2018, 29, 160–179. [Google Scholar] [CrossRef] [Green Version]
- Barrett, J.A.; Cai, H.; Miao, J.; Sun, L.; Murugesan, S.R.; Chan, T.A.; Chakiath, M.; Krishnan, S.; Einstein, R.; Lebel, F.; et al. A Synthetic Biology Rheoswitch Therapeutic System® for the Controlled Local Expression of IL-12 as an Immunotherapy for the Treatment of Cancer. Cell. Biol. Res. Ther. 2016, 5, 7. [Google Scholar]
- Hemmi, H.; Akira, S. TLR signalling and the function of dendritic cells. Chem. Immunol. Allergy 2005, 86, 120–135. [Google Scholar] [CrossRef]
- Adams, S. Toll-like receptor agonists in cancer therapy. Immunotherapy 2009, 1, 949–964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaczanowska, S.; Joseph, A.M.; Davila, E. TLR agonists: Our best frenemy in cancer immunotherapy. J. Leukoc. Biol. 2013, 93, 847–863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, L.; Wang, X.; Zhang, D. TLRs as a Promise Target Along With Immune Checkpoint Against Gastric Cancer. Front. Cell Dev. Biol. 2021, 8, 611444. [Google Scholar] [CrossRef] [PubMed]
- Reilley, M.J.; Morrow, B.; Ager, C.R.; Liu, A.; Hong, D.S.; Curran, M.A. TLR9 activation cooperates with T cell checkpoint blockade to regress poorly immunogenic melanoma. J. ImmunoTherapy Cancer 2019, 7, 323. [Google Scholar] [CrossRef]
- Pahlavanneshan, S.; Sayadmanesh, A.; Ebrahimiyan, H.; Basiri, M. Toll-Like Receptor-Based Strategies for Cancer Immunotherapy. J. Immunol. Res. 2021, 2021, 9912188. [Google Scholar] [CrossRef]
- Fares, C.M.; Van Allen, E.M.; Drake, C.G.; Allison, J.P.; Hu-Lieskovan, S. Mechanisms of Resistance to Immune Checkpoint Blockade: Why Does Checkpoint Inhibitor Immunotherapy Not Work for All Patients? Am. Soc. Clin. Oncol. Educ. Book 2019, 39, 147–164. [Google Scholar] [CrossRef]
- Wang, D.; Jiang, W.; Zhu, F.; Mao, X.; Agrawal, S. Modulation of the tumor microenvironment by intratumoral administration of IMO-2125, a novel TLR9 agonist, for cancer immunotherapy. Int. J. Oncol. 2018, 53, 1193–1203. [Google Scholar] [CrossRef] [Green Version]
- Haymaker, C.; Johnson, D.H.; Murthy, R.; Bentebibel, S.E.; Uemura, M.I.; Hudgens, C.W.; Safa, H.; James, M.; Andtbacka, R.H.I.; Johnson, D.B.; et al. Tilsotolimod with Ipilimumab Drives Tumor Responses in Anti-PD-1 Refractory Melanoma. Cancer Discov. 2021, 11, 1996–2013. [Google Scholar] [CrossRef]
- Wang, S.; Campos, J.; Gallotta, M.; Gong, M.; Crain, C.; Naik, E.; Coffman, R.L.; Guiducci, C. Intratumoral injection of a CpG oligonucleotide reverts resistance to PD-1 blockade by expanding multifunctional CD8+ T cells. Proc. Natl. Acad. Sci. USA 2016, 113, E7240–E7249. [Google Scholar] [CrossRef] [Green Version]
- Adamus, T.; Kortylewski, M. The revival of CpG oligonucleotide-based cancer immunotherapies. Contemp. Oncol. 2018, 22, 56–60. [Google Scholar] [CrossRef]
- Sabree, S.A.; Voigt, A.P.; Blackwell, S.E.; Vishwakarma, A.; Chimenti, M.S.; Salem, A.K.; Weiner, G.J. Direct and indirect immune effects of CMP-001, a virus-like particle containing a TLR9 agonist. J. Immunother. Cancer 2021, 9, e002484. [Google Scholar] [CrossRef] [PubMed]
- Davar, D.; Karunamurthy, A.; Hartman, D.; DeBlasio, R.; Chauvin, J.-M.; Ding, Q.; Pagliano, O.; Rose, A.; Kirkwood, J.; Zarour, H. 303 Phase II trial of neoadjuvant nivolumab (Nivo) and intra-tumoral (IT) CMP-001 in high-risk resectable melanoma (Neo-C-Nivo): Final results. J. ImmunoTherapy Cancer 2020, 8, A185–A186. [Google Scholar] [CrossRef]
- Kandimalla, E.R.; Nallagatla, S.; Anderson, B.R.; Kang, R. Abstract A136: AST-008, a novel TLR9 agonist SNA, induces abscopal antitumor effects in mouse tumor models. Cancer Immunol. Res. 2019, 7, A136. [Google Scholar] [CrossRef]
- Kivimae, S.; Hennessy, M.; Pena, R.; Kirksey, Y.; Nieves, W.; Quatch, P.; Cetz, J.; Ren, Z.; Cai, H.; Deng, B. Comprehensive antitumor immune activation by a novel TLR7/8 targeting agent NKTR-262 combined with CD122-biased immunostimulatory cytokine NKTR-214. Cancer Res. 2018, 78, 3755. [Google Scholar] [CrossRef]
- Diab, A.; Marcondes, M.; Kotzin, B.; Tagliaferri, M.A.; Hoch, U.; Li, Y.; Cattaruzza, F.; Zalevsky, J.; Brohl, A.S.; Brugarolas, J. Phase Ib: Preliminary clinical activity and immune activation for NKTR-262 [TLR 7/8 agonist] plus NKTR-214 [CD122-biased agonist] in patients (pts) with locally advanced or metastatic solid tumors (REVEAL Phase Ib/II Trial). Am. Soc. Clin. Oncol. 2019, 37 (Suppl. 8), 26. [Google Scholar] [CrossRef]
- Diab, A.; Curti, B.; Bilen, M.; Brohl, A.; Domingo-Musibay, E.; Borazanci, E.; Fanton, C.; Haglund, C.; Vimal, M.; Muhsin, M.; et al. REVEAL: Phase 1 dose-escalation study of NKTR-262, a novel TLR7/8 agonist, plus bempegaldesleukin: Local innate immune activation and systemic adaptive immune expansion for treating solid tumors. J. ImmunoTherapy Cancer 2020, 8, A224–A225. [Google Scholar] [CrossRef]
- Fellner, C. Ipilimumab (yervoy) prolongs survival in advanced melanoma: Serious side effects and a hefty price tag may limit its use. Pharm. Ther. 2012, 37, 503. [Google Scholar]
- O’Day, S.J.; Maio, M.; Chiarion-Sileni, V.; Gajewski, T.F.; Pehamberger, H.; Bondarenko, I.N.; Queirolo, P.; Lundgren, L.; Mikhailov, S.; Roman, L. Efficacy and safety of ipilimumab monotherapy in patients with pretreated advanced melanoma: A multicenter single-arm phase II study. Ann. Oncol. 2010, 21, 1712–1717. [Google Scholar] [CrossRef]
- Ray, A.; Williams, M.A.; Meek, S.M.; Bowen, R.C.; Grossmann, K.F.; Andtbacka, R.H.; Bowles, T.L.; Hyngstrom, J.R.; Leachman, S.A.; Grossman, D. A phase I study of intratumoral ipilimumab and interleukin-2 in patients with advanced melanoma. Oncotarget 2016, 7, 64390. [Google Scholar] [CrossRef] [Green Version]
- Hurtado, P.; Au Peh, C. LL-37 promotes rapid sensing of CpG oligodeoxynucleotides by B lymphocytes and plasmacytoid dendritic cells. J. Immunol. 2010, 184, 1425–1435. [Google Scholar] [CrossRef] [Green Version]
- Kyi, C.; Roudko, V.; Sabado, R.; Saenger, Y.; Loging, W.; Mandeli, J.; Thin, T.H.; Lehrer, D.; Donovan, M.; Posner, M.; et al. Therapeutic Immune Modulation against Solid Cancers with Intratumoral Poly-ICLC: A Pilot Trial. Clin. Cancer Res. 2018, 24, 4937–4948. [Google Scholar] [CrossRef] [Green Version]
- Johnson, D.H.; Bentebibel, S.E.; Lecagoonporn, S.; Bernatchez, C.; Haymaker, C.L.; Murthy, R.; Tam, A.; Yee, C.; Amaria, R.N.; Patel, S.P. Phase I/II dose escalation and expansion cohort safety and efficacy study of image guided intratumoral CD40 agonistic monoclonal antibody APX005M in combination with systemic pembrolizumab for treatment naive metastatic melanoma. Am. Soc. Clin. Oncol. 2018, 36 (Suppl. 15), TPS3133. [Google Scholar] [CrossRef]
- Figdor, C.G.; de Vries, I.J.M.; Lesterhuis, W.J.; Melief, C.J. Dendritic cell immunotherapy: Mapping the way. Nat. Med. 2004, 10, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Conrad, P.; Malouf, G.; Stacey, M. The Australian polidocanol (aethoxysklerol) study: Results at 2 years. Dermatol. Surg. 1995, 21, 334–336. [Google Scholar] [CrossRef] [PubMed]

| Clinical Trial Number | Phase | Regimen | Melanoma Population | Intralesional Dosing Schedule | Preliminary Results |
|---|---|---|---|---|---|
| NCT02557321 | 1b/2 | IT PV-10 + IV pembro vs. IV pembro alone | ICI-Naïve and ICI-refractory, unresectable, stage III–IV | Q3W x 5C | |
| NCT03474497 | 1/2 | IT IL-2 + IV ICI + hypofractionated XRT | PD-1/L1-refractory, stage IV | 2x/W x 4C | - |
| NCT03928275 | 2/3 | IT IL-2 vs. IT IL-2 + IT BCG | Stage IIIC-IVM1a | Q2W x 4C | - |
| NCT03132675 | 2 | IT Tavo-EP + IV pembro | Anti-PD-1-refractory, unresectable, stage III–IV | Days 1, 5, 8, Q6W, up to 18C |
|
| NCT02076646 | 1/2 | IT L19IL2 + IV DTIC vs. IV DTIC alone | Stage IV (M1a-b in phase 2) | Days 1, 8, 15 Q3W | - |
| NCT03567889 | 3 | IT Daromun + surgery + IV ID adjuvant vs. surgery + IV ID adjuvant | Neoadjuvant, resectable stage IIIB-C | Q1W, up to 4C | - |
| NCT02938299 | 3 | IT Daromun + surgery vs. surgery | Neoadjuvant, resectable stage IIIB-C | Q1W, up to 4C | - |
| NCT04427306 | 2 | IT T-VEC + surgery | Neoadjuvant, resectable, high-risk | Not specified | - |
| NCT04068181 | 2 | IT T-VEC + IV pembro | Anti-PD-1-refractory, unresectable, stage IIIB-IV | Q3W, up to 35C |
|
| NCT04330430 | 2 | IT T-VEC + IV nivo | Neoadjuvant, resectable stage IIIB-IVM1a | Q3W x 1C then Q2W x 3C | - |
| NCT03842943 | 2 | IN T-VEC + IV pembro | Neoadjuvant, clinically node-positive, resectable, stage III | Q3W up to 6 mos. | - |
| NCT02819843 | 2 | IT T-VEC ± XRT | Unresectable, stage IIIB-IV | Q3W x 1C then Q2W x 7C | - |
| NCT03555032 | 1/2 | IT T-VEC + ILP | Amenable to ILP, stage IIIB-IVM1b | Q2-3 Weeks x 3 doses | - |
| NCT03767348 | 1/2 | IT RP1 ± IV nivo | Stage IIIB-IVM1c | Not specified | |
| NCT04349436 | 1b/2 | IT RP1 | Treatment-refractory, locally advanced or metastatic, solid organ transplant recipients | Q2W | - |
| NCT04200040 | 2 | IT OrienX010 vs. IV DTIC | Treatment-naïve, unresectable, stage IIIB-IVM1b | Q2W | - |
| NCT05561491 | 2 | IT Oncos-102 ± IV balstilimab | Anti-PD-1-refractory, unresectable or metastatic | Not specified | - |
| NCT04725331 | 1/2a | IT BT-001 ± IV pembro | Locally advanced or metastatic | Not specified | - |
| NCT04152863 | 2 | IT & IV CVA21 + IV pembro vs. IV pembro | ICI-naïve, stage III-IV | Day 1, 3, 5, 8 Q4W x 1C, then Q3W x up to 7C | - |
| NCT04303169 | 1/2 | IT CVA21 + IV pembro + surgery vs. other investigational drugs + IV pembro + surgery | Neoadjuvant, treatment-naïve, resectable, stage IIIB-D | Not specified | - |
| NCT04577807 | 2 | IT PVSRIPO ± IV anti-PD-1 therapy | Anti-PD-1/L1-refractory, unresectable, stage III-IVM1b | Q1W x 7C then Q3-4W | - |
| NCT03190824 | 2a | IT OBP-301 | Unresectable, stage IIIB-IV | Q2W up to 13C | - |
| NCT04291105 | 2 | IT and IV VV1 + IV cemiplimab ± ipi | Anti-PD-1/L1-refractory, advanced or metastatic | Q4W x 1C, then Q3W | - |
| NCT03544723 | 2 | IT Ad-p53 + IV ID ICI | Recurrent or metastatic | Not specified | - |
| NCT01397708 | 1/2 | IT RheoSwitch | Unresectable, stage III-IV | Q3W up to 6 C |
|
| NCT04698187 | 2 | IT vidutolimod + IV nivo | Anti-PD-1-refractory, unresectable or metastatic | Q1W x 7C then Q3W | - |
| NCT04695977 | 2/3 | IT vidutolimod + IV nivo vs. IV nivo | Treatment-naïve, unresectable or metastatic | Q1W x 7C then Q3W | - |
| NCT04401995 | 2 | IT vidutolimod + IV nivo vs. IV nivo | Neoadjuvant, palpable nodal disease or nodal recurrence, stage IIIB-D | Q1W x 7C | - |
| NCT04708418 | 2 | IT vidutolimod + IV pembro + surgery vs. IV pembro + surgery | Neoadjuvant, resectable, stage III N1b-N3c | Day 1, 8, 15 Q3W x 2C then Q3W x 4C | - |
| NCT03684785 | 1b/2 | IT cavrotolimod + IV pembro | Anti-PD-1/L1-refractory, locally advanced or metastatic | Not specified |
|
| NCT02857569 | 1/2 | IT ipi + IV nivo vs. IV ipi + IV nivo | Unresectable, stage III-IV | Q3W up to 4C | - |
| NCT03755739 | 2/3 | IT ID ICIs vs. trans-arterial ID ICIs vs. IV ID ICIs | Advanced | Q3W | - |
| NCT02225366 | 1/2 | LL37 | Unresectable, stage IIIB-IVA | Q1W up to 8C | - |
| NCT02423863 | 2 | IT hiltonol + IM hiltonol ± IV anti-PD-1/L1 | Unresectable, advanced | IT Day 1, 3, 5 x 1W then IM 2x/W | - |
| NCT02706353 | 1/2 | IT APX005M + IV pembro | ICI-naïve, unresectable, stage III-IVM1c | Q3W up to 4C | - |
| NCT03325101 | 1b/2 | IT DCs + cryosurgery + IV pembro | Anti-PD-1/L1-refractory, unresectable, stage III-IV | Apheresis then Q3W x 2C | - |
| NCT03058289 | 1/2 | IT INT230-6 ± IV ICI | Treatment-refractory, metastatic | Q4W x 5C |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
DePalo, D.K.; Zager, J.S. Advances in Intralesional Therapy for Locoregionally Advanced and Metastatic Melanoma: Five Years of Progress. Cancers 2023, 15, 1404. https://doi.org/10.3390/cancers15051404
DePalo DK, Zager JS. Advances in Intralesional Therapy for Locoregionally Advanced and Metastatic Melanoma: Five Years of Progress. Cancers. 2023; 15(5):1404. https://doi.org/10.3390/cancers15051404
Chicago/Turabian StyleDePalo, Danielle K., and Jonathan S. Zager. 2023. "Advances in Intralesional Therapy for Locoregionally Advanced and Metastatic Melanoma: Five Years of Progress" Cancers 15, no. 5: 1404. https://doi.org/10.3390/cancers15051404