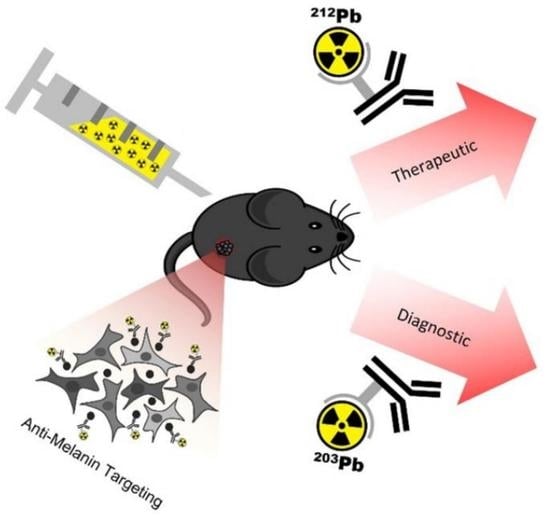
A Theranostic Approach to Imaging and Treating Melanoma with 203Pb/212Pb-Labeled Antibody Targeting Melanin
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Cancer Society. Melanoma of the Skin. Available online: https://cancerstatisticscenter.cancer.org/#!/cancer-site/Melanoma%20of%20the%20skin (accessed on 4 March 2022).
- National Cancer Institute. Cancer Statistics. Available online: https://www.cancer.gov/about-cancer/understanding/statistics#:~:text=The%20most%20common%20cancers%20(listed,endometrial%20cancer%2C%20leukemia%2C%20pancreatic%20cancer (accessed on 4 March 2022).
- Ragusa, F.; Ferrari, S.M.; Elia, G.; Paparo, S.R.; Balestri, E.; Botrini, C.; Patrizio, A.; Mazzi, V.; Guglielmi, G.; Foddis, R.; et al. Combination Strategies Involving Immune Checkpoint Inhibitors and Tyrosine Kinase or BRAF Inhibitors in Aggressive Thyroid Cancer. Int. J. Mol. Sci. 2022, 23, 5731. [Google Scholar] [CrossRef]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A guide to cancer immunotherapy: From T cell basic science to clinical practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef]
- Moser, J.C.; Wei, G.; Colonna, S.V.; Grossmann, K.F.; Patel, S.; Hyngstrom, J.R. Comparative-effectiveness of pembrolizumab vs. nivolumab for patients with metastatic melanoma. Acta Oncol. 2020, 59, 434–437. [Google Scholar] [CrossRef]
- Philips, G.K.; Atkins, M. Therapeutic uses of anti-PD-1 and anti-PD-L1 antibodies. Int. Immunol. 2014, 27, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Lelliott, E.J.; McArthur, G.A.; Oliaro, J.; Sheppard, K.E. Immunomodulatory Effects of BRAF, MEK, and CDK4/6 Inhibitors: Implications for Combining Targeted Therapy and Immune Checkpoint Blockade for the Treatment of Melanoma. Front. Immunol. 2021, 12, 661737. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Kim, K.B.; Schuchter, L.M.; Gonzalez, R.; Pavlick, A.C.; Weber, J.S.; McArthur, G.A.; Hutson, T.E.; Flaherty, K.T.; Moschos, S.J.; et al. BRIM-2: An open-label, multicenter phase II study of vemurafenib in previously treated patients with BRAF V600E mutation-positive metastatic melanoma. J. Clin. Oncol. 2011, 29, 8509. [Google Scholar] [CrossRef]
- Chapman, P.B.; Hauschild, A.; Robert, C.; Larkin, J.M.G.; Haanen, J.B.A.G.; Ribas, A.; Hogg, D.; O’Day, S.; Ascierto, P.A.; Testori, A.; et al. Phase III randomized, open-label, multicenter trial (BRIM3) comparing BRAF inhibitor vemurafenib with dacarbazine (DTIC) in patients with V600EBRAF-mutated melanoma. J. Clin. Oncol. 2011, 29, LBA4. [Google Scholar] [CrossRef]
- Dummer, R.; Ascierto, P.A.; Gogas, H.J.; Arance, A.; Mandala, M.; Liszkay, G.; Garbe, C.; Schadendorf, D.; Krajsova, I.; Gutzmer, R.; et al. Overall survival in patients with BRAF-mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018, 19, 1315–1327. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.J.H.; Malo, M.E.; Jiao, R.; Dadachova, E. Targeting Melanin in Melanoma with Radionuclide Therapy. Int. J. Mol. Sci. 2022, 23, 9520. [Google Scholar] [CrossRef]
- Dadachova, E.; Nosanchuk, J.D.; Shi, L.; Schweitzer, A.D.; Frenkel, A.; Nosanchuk, J.S.; Casadevall, A. Dead cells in melanoma tumors provide abundant antigen for targeted delivery of ionizing radiation by a mAb to melanin. Proc. Natl. Acad. Sci. USA 2004, 101, 14865–14870. [Google Scholar] [CrossRef]
- Jandl, T.; Revskaya, E.; Jiang, Z.; Harris, M.; Dorokhova, O.; Tsukrov, D.; Casadevall, A.; Dadachova, E. Melanoma stem cells in experimental melanoma are killed by radioimmunotherapy. Nucl. Med. Biol. 2013, 40, 177–181. [Google Scholar] [CrossRef]
- Allen, K.J.H.; Jiao, R.; Malo, M.E.; Frank, C.; Fisher, D.R.; Rickles, D.; Dadachova, E. Comparative Radioimmunotherapy of Experimental Melanoma with Novel Humanized Antibody to Melanin Labeled with 213Bismuth and 177Lutetium. Pharmaceutics 2019, 11, 348. [Google Scholar] [CrossRef] [Green Version]
- Couturier, O.; Supiot, S.; Degraef-Mougin, M.; Faivre-Chauvet, A.; Carlier, T.; Chatal, J.F.; Davodeau, F.; Cherel, M. Cancer radioimmunotherapy with alpha-emitting nuclides. Eur. J. Nucl. Med. 2005, 32, 601–614. [Google Scholar] [CrossRef] [PubMed]
- Poty, S.; Francesconi, L.C.; McDevitt, M.R.; Morris, M.J.; Lewis, J.S. α-Emitters for Radiotherapy: From Basic Radiochemistry to Clinical Studies—Part 2. Eur. J. Nucl. Med. 2018, 59, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Sgouros, G. Dosimetry, Radiobiology and Synthetic Lethality: Radiopharmaceutical Therapy (RPT) with Alpha-Particle-Emitters. Semin. Nucl. Med. 2020, 50, 124–132. [Google Scholar] [CrossRef]
- Li, M.; Sagastume, E.A.; Lee, D.; McAlister, D.; DeGraffenreid, A.J.; Olewine, K.R.; Graves, S.; Copping, R.; Mirzadeh, S.; Zimmerman, B.E.; et al. (203/212)Pb Theranostic Radiopharmaceuticals for Image-guided Radionuclide Therapy for Cancer. Curr. Med. Chem. 2020, 27, 7003–7031. [Google Scholar] [CrossRef]
- Nelson, B.J.B.; Wilson, J.; Schultz, M.K.; Andersson, J.D.; Wuest, F. High-yield cyclotron production of (203)Pb using a sealed (205)Tl solid target. Nucl. Med. Biol. 2023, 116–117, 108314. [Google Scholar] [CrossRef] [PubMed]
- Bryan, R.A.; Jiang, Z.; Jandl, T.; Strauss, J.; Koba, W.; Onyedika, C.; Morgenstern, A.; Bruchertseifer, F.; Epstein, A.L.; Dadachova, E. Treatment of experimental pancreatic cancer with 213-Bismuth-labeled chimeric antibody to single-strand DNA. Expert Rev. Anticancer. Ther. 2014, 4, 1243–1249. [Google Scholar] [CrossRef]
- Yong, K.J.; Milenic, D.E.; Baidoo, K.E.; Brechbiel, M.W. Cell Killing Mechanisms and Impact on Gene Expression by Gemcitabine and 212Pb-Trastuzumab Treatment in a Disseminated i.p. Tumor Model. PLoS ONE 2016, 11, e0159904. [Google Scholar] [CrossRef] [Green Version]
- Durand-Panteix, S.; Monteil, J.; Sage, M.; Garot, A.; Clavel, M.; Saidi, A.; Torgue, J.; Cogne, M.; Quelven, I. Preclinical study of 212Pb alpha-radioimmunotherapy targeting CD20 in non-Hodgkin lymphoma. Br. J. Cancer 2021, 125, 1657–1665. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pastan, I. High Shed Antigen Levels within Tumors: An Additional Barrier to lmmunoconjugate Therapy. Clin. Cancer Res. 2008, 14, 7981–7986. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, A.D.; Rakesh, V.; Revskaya, E.; Datta, A.; Casadevall, A.; Dadachova, E. Computational model predicts effective delivery of 188-Re-labeled melanin-binding antibody to metastatic melanoma tumors with wide range of melanin concentrations. Melanoma Res. 2007, 17, 291–303. [Google Scholar] [CrossRef]
- Tashima, T. Delivery of Drugs into Cancer Cells Using Antibody-Drug Conjugates Based on Receptor-Mediated Endocytosis and the Enhanced Permeability and Retention Effect. Antibodies 2022, 11, 78. [Google Scholar] [CrossRef] [PubMed]
- Milenic, D.E.; Garmestani, K.; Brady, E.D.; Albert, P.S.; Ma, D.; Abdulla, A.; Brechbiel, M.W. Targeting of HER2 antigen for the treatment of disseminated peritoneal disease. Clin. Cancer Res. 2004, 10, 7834–7841. [Google Scholar] [CrossRef] [Green Version]
- Kalinovsky, D.V.; Kibardin, A.V.; Kholodenko, I.V.; Svirshchevskaya, E.V.; Doronin, I.I.; Konovalova, M.V.; Grechikhina, M.V.; Rozov, F.N.; Larin, S.S.; Deyev, S.M.; et al. Therapeutic efficacy of antibody-drug conjugates targeting GD2-positive tumors. J. Immunother. Cancer 2022, 10, e004646. [Google Scholar] [CrossRef]
- D’Amico, L.; Menzel, U.; Prummer, M.; Müller, P.; Buchi, M.; Kashyap, A.; Haessler, U.; Yermanos, A.; Gébleux, R.; Briendl, M.; et al. A novel anti-HER2 anthracycline-based antibody-drug conjugate induces adaptive anti-tumor immunity and potentiates PD-1 blockade in breast cancer. J. Immunother. Cancer 2019, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, M.H.; Simonian, M.; Salimi, A.; Mirzadegan, E.; Sadeghi, N.; Nejadmoghaddam, M.-R.; Ebrahimnezhad, N.; Fazli, G.; Fatemi, R.; Bayat, A.-A.; et al. A novel ADC targeting cell surface fibromodulin in a mouse model of triple-negative breast cancer. Breast Cancer 2022, 29, 1121–1132. [Google Scholar] [CrossRef]
- Li, M.; Zhang, X.; Quinn, T.P.; Lee, D.; Liu, D.; Kunkel, F.; Zimmerman, B.E.; McAlister, D.; Olewein, K.; Menda, Y.; et al. Automated cassette-based production of high specific activity [(203/212)Pb]peptide-based theranostic radiopharmaceuticals for image-guided radionuclide therapy for cancer. Appl. Radiat. Isot. 2017, 127, 52–60. [Google Scholar] [CrossRef]
- Dos Santos, J.C.; Schäfer, M.; Bauder-Wüst, U.; Lehnert, W.; Leotta, K.; Morgenstern, A.; Kopka, K.; Haberkorn, U.; Mier, W.; Kratochwil, C. Development and dosimetry of 203Pb/212Pb-labelled PSMA ligands: Bringing “the lead” into PSMA-targeted alpha therapy? Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 1081–1091. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.R.; Minn, I.; Kumar, V.; Josefsson, A.; Lisok, A.; Brummet, M.; Chen, J.; Kiess, A.P.; Baidoo, K.; Brayton, C.; et al. Preclinical Evaluation of (203/212)Pb-Labeled Low-Molecular-Weight Compounds for Targeted Radiopharmaceutical Therapy of Prostate Cancer. J. Nucl. Med. 2020, 61, 80–88. [Google Scholar] [CrossRef]
- Li, M.; Liu, D.; Lee, D.; Kapoor, S.; Gibson-Corley, K.N.; Quinn, T.P.; Sagastume, E.A.; Mott, S.L.; Walsh, S.A.; Acevedo, M.R.; et al. Enhancing the Efficacy of Melanocortin 1 Receptor-Targeted Radiotherapy by Pharmacologically Upregulating the Receptor in Metastatic Melanoma. Mol. Pharm. 2019, 16, 3904–3915. [Google Scholar] [CrossRef]
- Yang, J.; Xu, J.; Cheuy, L.; Gonzalez, R.; Fisher, D.R.; Miao, Y. Evaluation of a Novel Pb-203-Labeled Lactam-Cyclized Alpha-Melanocyte-Stimulating Hormone Peptide for Melanoma Targeting. Mol. Pharm. 2019, 16, 1694–1702. [Google Scholar] [CrossRef]
- Miao, Y.; Figueroa, S.D.; Fisher, D.R.; Moore, H.A.; Testa, R.F.; Hoffman, T.J.; Quinn, T.P. 203Pb-labeled alpha-melanocyte-stimulating hormone peptide as an imaging probe for melanoma detection. J. Nucl. Med. 2008, 49, 823–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miao, Y.; Hylarides, M.; Fisher, D.R.; Shelton, T.; Moore, H.; Wester, D.W.; Fritzberg, A.R.; Winkelmann, C.T.; Hoffman, T.; Quinn, T.P. Melanoma therapy via peptide-targeted α-Radiation. Clin. Cancer Res. 2005, 11, 5616–5621. [Google Scholar] [CrossRef] [Green Version]
- Pikul, S.S., 2nd; Parks, N.J.; Schneider, P.D. In vitro killing of melanoma by liposome-delivered intracellular irradiation. Arch. Surg. 1987, 122, 1417–1420. [Google Scholar] [CrossRef]
- Meredith, R.; Torgue, J.; Shen, S.; Fisher, D.R.; Banaga, E.; Bunch, P.; Morgan, D.; Fan, J.; Straughn, J.M., Jr. Dose escalation and dosimetry of first-in-human α radioimmunotherapy with 212Pb-TCMC-trastuzumab. J. Nucl. Med. 2014, 55, 1636–1642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meredith, R.F.; Torgue, J.; Azure, M.T.; Shen, S.; Saddekni, S.; Banaga, E.; Carlise, R.; Bunch, P.; Yoder, D.; Alvarez, R. Pharmacokinetics and imaging of 212Pb-TCMC-trastuzumab after intraperitoneal administration in ovarian cancer patients. Cancer Biother. Radiopharm. 2014, 29, 12–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiao, R.; Allen, K.J.H.; Malo, M.E.; Yilmaz, O.; Wilson, J.; Nelson, B.J.B.; Wuest, F.; Dadachova, E. A Theranostic Approach to Imaging and Treating Melanoma with 203Pb/212Pb-Labeled Antibody Targeting Melanin. Cancers 2023, 15, 3856. https://doi.org/10.3390/cancers15153856
Jiao R, Allen KJH, Malo ME, Yilmaz O, Wilson J, Nelson BJB, Wuest F, Dadachova E. A Theranostic Approach to Imaging and Treating Melanoma with 203Pb/212Pb-Labeled Antibody Targeting Melanin. Cancers. 2023; 15(15):3856. https://doi.org/10.3390/cancers15153856
Chicago/Turabian StyleJiao, Rubin, Kevin J. H. Allen, Mackenzie E. Malo, Orhan Yilmaz, John Wilson, Bryce J. B. Nelson, Frank Wuest, and Ekaterina Dadachova. 2023. "A Theranostic Approach to Imaging and Treating Melanoma with 203Pb/212Pb-Labeled Antibody Targeting Melanin" Cancers 15, no. 15: 3856. https://doi.org/10.3390/cancers15153856