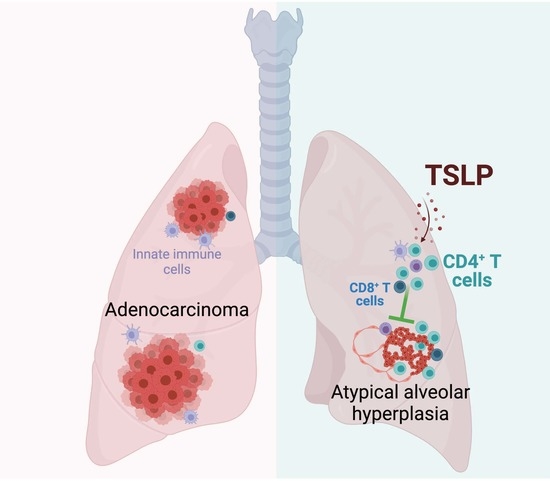
Thymic Stromal Lymphopoietin Induction Suppresses Lung Cancer Development
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Statistical Analysis
2.3. Study Approval
2.4. Spontaneous Lung Cancer Studies
2.5. Histology and Immunofluorescence
2.6. CD4+ T Cell Depletion
2.7. Flow Cytometry
3. Results
3.1. TSLP Induction Reduces Tumor Burden in a Mouse Model of Spontaneous Lung Carcinogenesis
3.2. TSLP Induction Arrests Tumor Development at an Early Adenoma-like Stage
3.3. Tslptg KrasG12D Lungs and Tumors Are Highly Infiltrated by CD4+ T Cells
3.4. TSLP-Activated CD4+ T Cells Are Required for Suppressing Lung Carcinogenesis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Schabath, M.B.; Cote, M.L. Cancer Progress and Priorities: Lung Cancer. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1563–1579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, H.; Suzuki, A.; Tatematsu, T.; Shitara, M.; Hikosaka, Y.; Okuda, K.; Moriyama, S.; Yano, M.; Fujii, Y. Prognosis of recurrent non-small cell lung cancer following complete resection. Oncol. Lett. 2014, 7, 1300–1304. [Google Scholar] [CrossRef] [Green Version]
- Ramos-Casals, M.; Brahmer, J.R.; Callahan, M.K.; Flores-Chavez, A.; Keegan, N.; Khamashta, M.A.; Lambotte, O.; Mariette, X.; Prat, A.; Suarez-Almazor, M.E. Immune-related adverse events of checkpoint inhibitors. Nat. Rev. Dis. Primers 2020, 6, 38. [Google Scholar] [CrossRef]
- Finn, O.J. The dawn of vaccines for cancer prevention. Nat. Rev. Immunol. 2018, 18, 183–194. [Google Scholar] [CrossRef]
- Nagahashi, M.; Sato, S.; Yuza, K.; Shimada, Y.; Ichikawa, H.; Watanabe, S.; Takada, K.; Okamoto, T.; Okuda, S.; Lyle, S.; et al. Common driver mutations and smoking history affect tumor mutation burden in lung adenocarcinoma. J. Surg. Res. 2018, 230, 181–185. [Google Scholar] [CrossRef] [Green Version]
- Yarchoan, M.; Hopkins, A.; Jaffee, E.M. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N. Engl. J. Med. 2017, 377, 2500–2501. [Google Scholar] [CrossRef]
- Demehri, S.; Turkoz, A.; Manivasagam, S.; Yockey, L.J.; Turkoz, M.; Kopan, R. Elevated epidermal thymic stromal lymphopoietin levels establish an antitumor environment in the skin. Cancer Cell 2012, 22, 494–505. [Google Scholar] [CrossRef] [Green Version]
- Demehri, S.; Cunningham, T.J.; Manivasagam, S.; Ngo, K.H.; Moradi Tuchayi, S.; Reddy, R.; Meyers, M.A.; DeNardo, D.G.; Yokoyama, W.M. Thymic stromal lymphopoietin blocks early stages of breast carcinogenesis. J. Clin. Investig. 2016, 126, 1458–1470. [Google Scholar] [CrossRef] [Green Version]
- Varricchi, G.; Pecoraro, A.; Marone, G.; Criscuolo, G.; Spadaro, G.; Genovese, A.; Marone, G. Thymic Stromal Lymphopoietin Isoforms, Inflammatory Disorders, and Cancer. Front. Immunol. 2018, 9, 1595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Zein, M.; Parent, M.E.; Ka, K.; Siemiatycki, J.; St-Pierre, Y.; Rousseau, M.C. History of asthma or eczema and cancer risk among men: A population-based case-control study in Montreal, Quebec, Canada. Ann. Allergy Asthma Immunol. 2010, 104, 378–384. [Google Scholar] [CrossRef] [PubMed]
- El-Zein, M.; Parent, M.E.; Siemiatycki, J.; Rousseau, M.C. History of allergic diseases and lung cancer risk. Ann. Allergy Asthma Immunol. 2014, 112, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Cianferoni, A.; Spergel, J. The importance of TSLP in allergic disease and its role as a potential therapeutic target. Expert Rev. Clin. Immunol. 2014, 10, 1463–1474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- West, E.E.; Kashyap, M.; Leonard, W.J. TSLP: A Key Regulator of Asthma Pathogenesis. Drug Discov. Today Dis. Mech. 2012, 9, e83–e88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Zhao, H.; Yu, J.; Su, Y.; Cao, S.; An, X.; Ren, X. Increased prevalence of regulatory T cells in the lung cancer microenvironment: A role of thymic stromal lymphopoietin. Cancer Immunol. Immunother. 2011, 60, 1587–1596. [Google Scholar] [CrossRef]
- Pedroza-Gonzalez, A.; Xu, K.; Wu, T.C.; Aspord, C.; Tindle, S.; Marches, F.; Gallegos, M.; Burton, E.C.; Savino, D.; Hori, T.; et al. Thymic stromal lymphopoietin fosters human breast tumor growth by promoting type 2 inflammation. J. Exp. Med. 2011, 208, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Olkhanud, P.B.; Rochman, Y.; Bodogai, M.; Malchinkhuu, E.; Wejksza, K.; Xu, M.; Gress, R.E.; Hesdorffer, C.; Leonard, W.J.; Biragyn, A. Thymic Stromal Lymphopoietin Is a Key Mediator of Breast Cancer Progression. J. Immunol. 2011, 186, 5656–5662. [Google Scholar] [CrossRef] [PubMed]
- De Monte, L.; Reni, M.; Tassi, E.; Clavenna, D.; Papa, I.; Recalde, H.; Braga, M.; Di Carlo, V.; Doglioni, C.; Protti, M.P. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J. Exp. Med. 2011, 208, 469–478. [Google Scholar] [CrossRef]
- Kuan, E.L.; Ziegler, S.F. A tumor-myeloid cell axis, mediated via the cytokines IL-1alpha and TSLP, promotes the progression of breast cancer. Nat. Immunol. 2018, 19, 366–374. [Google Scholar] [CrossRef]
- Di Piazza, M.; Nowell, C.S.; Koch, U.; Durham, A.D.; Radtke, F. Loss of Cutaneous TSLP-Dependent Immune Responses Skews the Balance of Inflammation from Tumor Protective to Tumor Promoting. Cancer Cell 2012, 22, 479–493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, L.; Mercer, K.; Greenbaum, D.; Bronson, R.T.; Crowley, D.; Tuveson, D.A.; Jacks, T. Somatic activation of the K-ras oncogene causes early onset lung cancer in mice. Nature 2001, 410, 1111–1116. [Google Scholar] [CrossRef]
- Mori, M.; Rao, S.K.; Popper, H.H.; Cagle, P.T.; Fraire, A.E. Atypical adenomatous hyperplasia of the lung: A probable forerunner in the development of adenocarcinoma of the lung. Mod. Pathol. 2001, 14, 72–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kerr, K.M. Pulmonary preinvasive neoplasia. J. Clin. Pathol. 2001, 54, 257–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeNardo, D.G.; Barreto, J.B.; Andreu, P.; Vasquez, L.; Tawfik, D.; Kolhatkar, N.; Coussens, L.M. CD4+ T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell 2009, 16, 91–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.J.; Cantor, H. CD4 T-cell subsets and tumor immunity: The helpful and the not-so-helpful. Cancer Immunol. Res. 2014, 2, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Domagala-Kulawik, J. The role of the immune system in non-small cell lung carcinoma and potential for therapeutic intervention. Transl. Lung Cancer Res. 2015, 4, 177–190. [Google Scholar] [CrossRef]
- Gao, G.; Liao, W.; Ma, Q.; Zhang, B.; Chen, Y.; Wang, Y. KRAS G12D mutation predicts lower TMB and drives immune suppression in lung adenocarcinoma. Lung Cancer 2020, 149, 41–45. [Google Scholar] [CrossRef]
- Lavin, Y.; Kobayashi, S.; Leader, A.; Amir, E.D.; Elefant, N.; Bigenwald, C.; Remark, R.; Sweeney, R.; Becker, C.D.; Levine, J.H.; et al. Innate Immune Landscape in Early Lung Adenocarcinoma by Paired Single-Cell Analyses. Cell 2017, 169, 750–765.e717. [Google Scholar] [CrossRef] [Green Version]
- Yasukawa, M.; Sawabata, N.; Kawaguchi, T.; Kawai, N.; Nakai, T.; Ohbayashi, C.; Taniguchi, S. Histological Grade: Analysis of Prognosis of Non-small Cell Lung Cancer After Complete Resection. Vivo 2018, 32, 1505–1512. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Aubry, M.C.; Deschamps, C.; Marks, R.S.; Okuno, S.H.; Williams, B.A.; Sugimura, H.; Pankratz, V.S.; Yang, P. Histologic grade is an independent prognostic factor for survival in non-small cell lung cancer: An analysis of 5018 hospital- and 712 population-based cases. J. Thorac. Cardiovasc. Surg. 2006, 131, 1014–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, R.; Celis, E. Multiple roles for CD4+ T cells in anti-tumor immune responses. Immunol. Rev. 2008, 222, 129–144. [Google Scholar] [CrossRef] [PubMed]
- Zander, R.; Schauder, D.; Xin, G.; Nguyen, C.; Wu, X.; Zajac, A.; Cui, W. CD4+ T Cell Help Is Required for the Formation of a Cytolytic CD8+ T Cell Subset that Protects against Chronic Infection and Cancer. Immunity 2019, 51, 1028–1042.e1024. [Google Scholar] [CrossRef] [PubMed]
- Tay, R.E.; Richardson, E.K.; Toh, H.C. Revisiting the role of CD4+ T cells in cancer immunotherapy-new insights into old paradigms. Cancer Gene Ther. 2021, 28, 5–17. [Google Scholar] [CrossRef]
- Alspach, E.; Lussier, D.M.; Miceli, A.P.; Kizhvatov, I.; DuPage, M.; Luoma, A.M.; Meng, W.; Lichti, C.F.; Esaulova, E.; Vomund, A.N.; et al. MHC-II neoantigens shape tumour immunity and response to immunotherapy. Nature 2019, 574, 696–701. [Google Scholar] [CrossRef]
- Liu, M.; Kuo, F.; Capistrano, K.J.; Kang, D.; Nixon, B.G.; Shi, W.; Chou, C.; Do, M.H.; Stamatiades, E.G.; Gao, S.; et al. TGF-beta suppresses type 2 immunity to cancer. Nature 2020, 587, 115–120. [Google Scholar] [CrossRef]
- Kagamu, H.; Kitano, S.; Yamaguchi, O.; Yoshimura, K.; Horimoto, K.; Kitazawa, M.; Fukui, K.; Shiono, A.; Mouri, A.; Nishihara, F.; et al. CD4+ T-cell Immunity in the Peripheral Blood Correlates with Response to Anti-PD-1 Therapy. Cancer Immunol. Res. 2020, 8, 334–344. [Google Scholar] [CrossRef] [Green Version]
- Laheurte, C.; Dosset, M.; Vernerey, D.; Boullerot, L.; Gaugler, B.; Gravelin, E.; Kaulek, V.; Jacquin, M.; Cuche, L.; Eberst, G.; et al. Distinct prognostic value of circulating anti-telomerase CD4+ Th1 immunity and exhausted PD-1+/TIM-3+ T cells in lung cancer. Br. J. Cancer 2019, 121, 405–416. [Google Scholar] [CrossRef] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guennoun, R.; Hojanazarova, J.; Trerice, K.E.; Azin, M.; McGoldrick, M.T.; Schiferle, E.B.; Stover, M.P.; Demehri, S. Thymic Stromal Lymphopoietin Induction Suppresses Lung Cancer Development. Cancers 2022, 14, 2173. https://doi.org/10.3390/cancers14092173
Guennoun R, Hojanazarova J, Trerice KE, Azin M, McGoldrick MT, Schiferle EB, Stover MP, Demehri S. Thymic Stromal Lymphopoietin Induction Suppresses Lung Cancer Development. Cancers. 2022; 14(9):2173. https://doi.org/10.3390/cancers14092173
Chicago/Turabian StyleGuennoun, Ranya, Jennet Hojanazarova, Kathryn E. Trerice, Marjan Azin, Matthew T. McGoldrick, Erik B. Schiferle, Michael P. Stover, and Shadmehr Demehri. 2022. "Thymic Stromal Lymphopoietin Induction Suppresses Lung Cancer Development" Cancers 14, no. 9: 2173. https://doi.org/10.3390/cancers14092173