Overcoming Therapy Resistance in Colon Cancer by Drug Repurposing
Abstract
:Simple Summary
Abstract
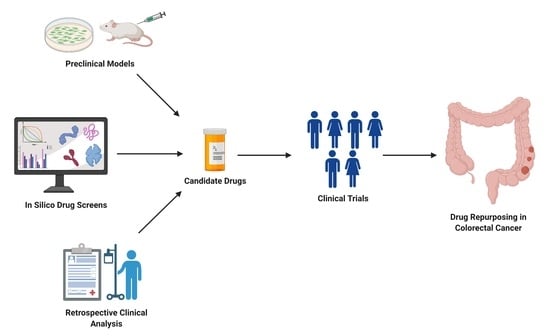
1. Introduction
2. Repurposing Approved Drugs in Colon Cancer
2.1. Anti-Hypertensives and Anti-Arrhythmic Drugs
2.2. Nonsteroidal Anti-Inflammatory Drugs
2.3. Anti-Hyperlipidemic Drugs
2.4. Anti-Diabetic Drugs
2.5. Anti-Helminthic Drugs
2.6. Anti-Retroviral Drugs
2.7. Anti-Microbials
2.8. Others
3. Clinical Trials on Drug Repurposing in Colon Cancer
4. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef]
- Wild, C.; Weiderpass, E.; Stewart, B.W. World Cancer Report: Cancer Research for Cancer Prevention. Cancer Control 2020, 199, 512. [Google Scholar]
- Guren, M.G. The global challenge of colorectal cancer. Lancet Gastroenterol. Hepatol. 2019, 4, 894–895. [Google Scholar] [CrossRef] [Green Version]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Recio-Boiles, A.C. Colon Cancer; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Itatani, Y.; Kawada, K. Treatment of Elderly Patients with Colorectal Cancer. BioMed Res. Int. 2018, 2018, 2176056. [Google Scholar] [CrossRef] [Green Version]
- Mármol, I.; Sánchez-de-Diego, C.; Pradilla Dieste, A.; Cerrada, E.; Rodriguez Yoldi, M.J. Colorectal Carcinoma: A General Overview and Future Perspectives in Colorectal Cancer. Int. J. Mol. Sci. 2017, 18, 197. [Google Scholar] [CrossRef] [Green Version]
- Van der Jeught, K.; Xu, H.C.; Li, Y.J.; Lu, X.B.; Ji, G. Drug resistance and new therapies in colorectal cancer. World J. Gastroenterol. 2018, 24, 3834–3848. [Google Scholar] [CrossRef]
- Das, P.K.; Islam, F.; Lam, A.K. The Roles of Cancer Stem Cells and Therapy Resistance in Colorectal Carcinoma. Cells 2020, 9, 1392. [Google Scholar] [CrossRef]
- Golshani, G.; Zhang, Y. Advances in immunotherapy for colorectal cancer: A review. Ther. Adv. Gastroenterol. 2020, 13, 1756284820917527. [Google Scholar] [CrossRef]
- Xie, Y.H.; Chen, Y.X.; Fang, J.Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct. Target. Ther. 2020, 5, 22. [Google Scholar] [CrossRef]
- Hammond, W.A.; Swaika, A.; Mody, K. Pharmacologic resistance in colorectal cancer: A review. Ther. Adv. Med. Oncol. 2016, 8, 57–84. [Google Scholar] [CrossRef] [Green Version]
- Giampieri, R.; Cantini, L.; Giglio, E.; Bittoni, A.; Lanese, A.; Crocetti, S.; Pecci, F.; Copparoni, C.; Meletani, T.; Lenci, E.; et al. Impact of Polypharmacy for Chronic Ailments in Colon Cancer Patients: A Review Focused on Drug Repurposing. Cancers 2020, 12, 2724. [Google Scholar] [CrossRef]
- Jourdan, J.P.; Bureau, R.; Rochais, C.; Dallemagne, P. Drug repositioning: A brief overview. J. Pharm. Pharmacol. 2020, 72, 1145–1151. [Google Scholar] [CrossRef] [Green Version]
- Nowak-Sliwinska, P.; Scapozza, L.; Ruiz i Altaba, A. Drug repurposing in oncology: Compounds, pathways, phenotypes and computational approaches for colorectal cancer. Biochim. Biophys. Acta Rev. Cancer 2019, 1871, 434–454. [Google Scholar] [CrossRef]
- Kumar, S.K.; Callander, N.S.; Adekola, K.; Anderson, L.; Baljevic, M.; Campagnaro, E.; Castillo, J.J.; Chandler, J.C.; Costello, C.; Efebera, Y.; et al. Multiple Myeloma, Version 3.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. JNCCN 2020, 18, 1685–1717. [Google Scholar] [CrossRef]
- Upputuri, B.; Pallapati, M.S.; Tarwater, P. Thalidomide in the treatment of erythema nodosum leprosum (ENL) in an outpatient setting: A five-year retrospective analysis from a leprosy referral centre in India. PLoS Neglected Trop. Dis. 2020, 14, e0008678. [Google Scholar] [CrossRef]
- Fink, H.A.; Mac Donald, R.; Rutks, I.R.; Nelson, D.B.; Wilt, T.J. Sildenafil for male erectile dysfunction: A systematic review and meta-analysis. Arch. Intern. Med. 2002, 162, 1349–1360. [Google Scholar] [CrossRef] [Green Version]
- Barnett, C.F.; Machado, R.F. Sildenafil in the treatment of pulmonary hypertension. Vasc. Health Risk Manag. 2006, 2, 411–422. [Google Scholar] [CrossRef]
- Institute of Medicine (US) Forum on Drug Discovery, Development, and Translation. Transforming Clinical Research in the United States: Challenges and Opportunities: Workshop Summary; National Academies Press (US): Washington, DC, USA, 2010. [Google Scholar]
- Xue, H.; Li, J.; Xie, H.; Wang, Y. Review of Drug Repositioning Approaches and Resources. Int. J. Biol. Sci. 2018, 14, 1232–1244. [Google Scholar] [CrossRef] [Green Version]
- U.S. Food and Drug Administration. Step 3: Clinical Research. Available online: https://www.fda.gov/patients/drug-development-process/step-3-clinical-research (accessed on 30 November 2021).
- Deotarse, P.J.A.; Baile, M.; Kohle, N.; Kulkarni, A. Drug Repurposing: A Review. Int. J. Pharm. Res. Rev. 2015, 4, 51–58. [Google Scholar]
- Parvathaneni, V.; Kulkarni, N.S.; Muth, A.; Gupta, V. Drug repurposing: A promising tool to accelerate the drug discovery process. Drug Discov. Today 2019, 24, 2076–2085. [Google Scholar] [CrossRef]
- Moffat, J.G.; Vincent, F.; Lee, J.A.; Eder, J.; Prunotto, M. Opportunities and challenges in phenotypic drug discovery: An industry perspective. Nat. Rev. Drug Discov. 2017, 16, 531–543. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- Zannad, F.; Ferreira, J.P.; Pocock, S.J.; Anker, S.D.; Butler, J.; Filippatos, G.; Brueckmann, M.; Ofstad, A.P.; Pfarr, E.; Jamal, W.; et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: A meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet 2020, 396, 819–829. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [Green Version]
- U.S. Food and Drug Administration. FDA Grants Accelerated Approval for Alzheimer’s Drug. Available online: https://www.fda.gov/news-events/press-announcements/fda-grants-accelerated-approval-alzheimers-drug (accessed on 30 November 2021).
- Paul, S.M.; Mytelka, D.S.; Dunwiddie, C.T.; Persinger, C.C.; Munos, B.H.; Lindborg, S.R.; Schacht, A.L. How to improve R&D productivity: The pharmaceutical industry’s grand challenge. Nat. Rev. Drug Discov. 2010, 9, 203–214. [Google Scholar] [CrossRef]
- Jacquemet, G.; Baghirov, H.; Georgiadou, M.; Sihto, H. L-type calcium channels regulate filopodia stability and cancer cell invasion downstream of integrin signalling. Nat. Commun. 2016, 7, 13297. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Zhou, L.; Xie, N.; Nice, E.C.; Zhang, T.; Cui, Y.; Huang, C. Overcoming cancer therapeutic bottleneck by drug repurposing. Signal Transduct. Target. Ther. 2020, 5, 113. [Google Scholar] [CrossRef]
- Lord, S.R.; Cheng, W.C.; Liu, D.; Gaude, E.; Haider, S.; Metcalf, T.; Patel, N.; Teoh, E.J.; Gleeson, F.; Bradley, K.; et al. Integrated Pharmacodynamic Analysis Identifies Two Metabolic Adaption Pathways to Metformin in Breast Cancer. Cell Metab. 2018, 28, 679–688.e674. [Google Scholar] [CrossRef] [Green Version]
- Jarada, T.N.; Rokne, J.G.; Alhajj, R. A review of computational drug repositioning: Strategies, approaches, opportunities, challenges, and directions. J. Cheminform. 2020, 12, 46. [Google Scholar] [CrossRef]
- Liu, X.; Zhu, F.; Ma, X.H.; Shi, Z.; Yang, S.Y.; Wei, Y.Q.; Chen, Y.Z. Predicting targeted polypharmacology for drug repositioning and multi- target drug discovery. Curr. Med. Chem. 2013, 20, 1646–1661. [Google Scholar] [CrossRef]
- Raynal, N.J.; Da Costa, E.M.; Lee, J.T.; Gharibyan, V.; Ahmed, S.; Zhang, H.; Sato, T.; Malouf, G.G.; Issa, J.J. Repositioning FDA-Approved Drugs in Combination with Epigenetic Drugs to Reprogram Colon Cancer Epigenome. Mol. Cancer Ther. 2017, 16, 397–407. [Google Scholar] [CrossRef] [Green Version]
- Kubota, M.; Shimizu, M.; Sakai, H.; Yasuda, Y.; Ohno, T.; Kochi, T.; Tsurumi, H.; Tanaka, T.; Moriwaki, H. Renin-angiotensin system inhibitors suppress azoxymethane-induced colonic preneoplastic lesions in C57BL/KsJ-db/db obese mice. Biochem. Biophys. Res. Commun. 2011, 410, 108–113. [Google Scholar] [CrossRef]
- Kedika, R.; Patel, M.; Pena Sahdala, H.N.; Mahgoub, A.; Cipher, D.; Siddiqui, A.A. Long-term use of angiotensin converting enzyme inhibitors is associated with decreased incidence of advanced adenomatous colon polyps. J. Clin. Gastroenterol. 2011, 45, e12–e16. [Google Scholar] [CrossRef]
- Nuevo-Tapioles, C.; Santacatterina, F.; Stamatakis, K.; Núñez de Arenas, C.; Gómez de Cedrón, M.; Formentini, L.; Cuezva, J.M. Coordinate β-adrenergic inhibition of mitochondrial activity and angiogenesis arrest tumor growth. Nat. Commun. 2020, 11, 3606. [Google Scholar] [CrossRef]
- Dovizio, M.; Tacconelli, S.; Sostres, C.; Ricciotti, E.; Patrignani, P. Mechanistic and pharmacological issues of aspirin as an anticancer agent. Pharmaceuticals 2012, 5, 1346–1371. [Google Scholar] [CrossRef] [Green Version]
- Thun, M.J.; Jacobs, E.J.; Patrono, C. The role of aspirin in cancer prevention. Nat. Rev. Clin. Oncol. 2012, 9, 259–267. [Google Scholar] [CrossRef]
- Kopp, H.G.; Placke, T.; Salih, H.R. Platelet-derived transforming growth factor-beta down-regulates NKG2D thereby inhibiting natural killer cell antitumor reactivity. Cancer Res. 2009, 69, 7775–7783. [Google Scholar] [CrossRef] [Green Version]
- Chan, A.T.; Ogino, S.; Fuchs, C.S. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N. Engl. J. Med. 2007, 356, 2131–2142. [Google Scholar] [CrossRef]
- Xu, X.T.; Hu, W.T.; Zhou, J.Y.; Tu, Y. Celecoxib enhances the radiosensitivity of HCT116 cells in a COX-2 independent manner by up-regulating BCCIP. Am. J. Transl. Res. 2017, 9, 1088–1100. [Google Scholar]
- Bertagnolli, M.M.; Eagle, C.J.; Zauber, A.G.; Redston, M.; Solomon, S.D.; Kim, K.; Tang, J.; Rosenstein, R.B.; Wittes, J.; Corle, D.; et al. Celecoxib for the prevention of sporadic colorectal adenomas. N. Engl. J. Med. 2006, 355, 873–884. [Google Scholar] [CrossRef]
- Juneja, M.; Kobelt, D.; Walther, W.; Voss, C.; Smith, J.; Specker, E.; Neuenschwander, M.; Gohlke, B.O.; Dahlmann, M.; Radetzki, S.; et al. Statin and rottlerin small-molecule inhibitors restrict colon cancer progression and metastasis via MACC1. PLoS Biol. 2017, 15, e2000784. [Google Scholar] [CrossRef] [Green Version]
- Qi, J.H.; Wei, J.N.; Zhang, Z.J.; Dong, L.; Zhang, L.; Mao, Y.Y.; Lei, L.J.; Hu, X.Q.; Bai, W.Q. [A Meta-analysis on association between statins and colorectal cancer]. Zhonghua Liu Xing Bing Xue Za Zhi 2021, 42, 343–350. [Google Scholar] [CrossRef]
- Slattery, M.L.; Herrick, J.S.; Lundgreen, A.; Fitzpatrick, F.A.; Curtin, K.; Wolff, R.K. Genetic variation in a metabolic signaling pathway and colon and rectal cancer risk: mTOR, PTEN, STK11, RPKAA1, PRKAG2, TSC1, TSC2, PI3K and Akt1. Carcinogenesis 2010, 31, 1604–1611. [Google Scholar] [CrossRef] [Green Version]
- Vial, G.; Detaille, D.; Guigas, B. Role of Mitochondria in the Mechanism(s) of Action of Metformin. Front. Endocrinol. 2019, 10, 294. [Google Scholar] [CrossRef] [Green Version]
- Zi, F.; Zi, H.; Li, Y.; He, J.; Shi, Q.; Cai, Z. Metformin and cancer: An existing drug for cancer prevention and therapy. Oncol. Lett. 2018, 15, 683–690. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.T.; Ung, T.T.; Li, S.; Lian, S.; Xia, Y.; Park, S.Y.; Do Jung, Y. Metformin inhibits lithocholic acid-induced interleukin 8 upregulation in colorectal cancer cells by suppressing ROS production and NF-kB activity. Sci. Rep. 2019, 9, 2003. [Google Scholar] [CrossRef] [Green Version]
- Saber, M.M.; Galal, M.A.; Ain-Shoka, A.A.; Shouman, S.A. Combination of metformin and 5-aminosalicylic acid cooperates to decrease proliferation and induce apoptosis in colorectal cancer cell lines. BMC Cancer 2016, 16, 126. [Google Scholar] [CrossRef] [Green Version]
- Okada, J.; Yamada, E.; Saito, T.; Yokoo, H.; Osaki, A.; Shimoda, Y.; Ozawa, A.; Nakajima, Y.; Pessin, J.E.; Okada, S.; et al. Dapagliflozin Inhibits Cell Adhesion to Collagen I and IV and Increases Ectodomain Proteolytic Cleavage of DDR1 by Increasing ADAM10 Activity. Molecules 2020, 25, 495. [Google Scholar] [CrossRef] [Green Version]
- Saito, T.; Okada, S.; Yamada, E.; Shimoda, Y.; Osaki, A.; Tagaya, Y.; Shibusawa, R.; Okada, J.; Yamada, M. Effect of dapagliflozin on colon cancer cell [Rapid Communication]. Endocr. J. 2015, 62, 1133–1137. [Google Scholar] [CrossRef] [Green Version]
- Nygren, P.; Fryknäs, M.; Agerup, B.; Larsson, R. Repositioning of the anthelmintic drug mebendazole for the treatment for colon cancer. J. Cancer Res. Clin. Oncol. 2013, 139, 2133–2140. [Google Scholar] [CrossRef] [Green Version]
- Williamson, T.; Bai, R.Y.; Staedtke, V.; Huso, D.; Riggins, G.J. Mebendazole and a non-steroidal anti-inflammatory combine to reduce tumor initiation in a colon cancer preclinical model. Oncotarget 2016, 7, 68571–68584. [Google Scholar] [CrossRef] [Green Version]
- Sack, U.; Walther, W.; Scudiero, D.; Selby, M.; Kobelt, D.; Lemm, M.; Fichtner, I.; Schlag, P.M.; Shoemaker, R.H.; Stein, U. Novel effect of antihelminthic Niclosamide on S100A4-mediated metastatic progression in colon cancer. J. Natl. Cancer Inst. 2011, 103, 1018–1036. [Google Scholar] [CrossRef] [Green Version]
- Osada, T.; Chen, M.; Yang, X.Y.; Spasojevic, I.; Vandeusen, J.B.; Hsu, D.; Clary, B.M.; Clay, T.M.; Chen, W.; Morse, M.A.; et al. Antihelminth compound niclosamide downregulates Wnt signaling and elicits antitumor responses in tumors with activating APC mutations. Cancer Res. 2011, 71, 4172–4182. [Google Scholar] [CrossRef] [Green Version]
- Sherif, D.A.; Makled, M.N.; Suddek, G.M. The HIV reverse transcriptase inhibitor Tenofovir suppressed DMH/HFD-induced colorectal cancer in Wistar rats. Fundam. Clin. Pharmacol. 2021, 35, 940–954. [Google Scholar] [CrossRef]
- Brown, T.; Sigurdson, E.; Rogatko, A.; Broccoli, D. Telomerase inhibition using azidothymidine in the HT-29 colon cancer cell line. Ann. Surg. Oncol. 2003, 10, 910–915. [Google Scholar] [CrossRef]
- Fang, X.; Hu, T.; Yin, H.; Yang, J.; Tang, W.; Hu, S.; Xu, X. Differences in telomerase activity and the effects of AZT in aneuploid and euploid cells in colon cancer. Int. J. Oncol. 2017, 51, 525–532. [Google Scholar] [CrossRef]
- Hecht, M.; Harrer, T.; Büttner, M.; Schwegler, M.; Erber, S.; Fietkau, R.; Distel, L.V. Cytotoxic effect of efavirenz is selective against cancer cells and associated with the cannabinoid system. AIDS 2013, 27, 2031–2040. [Google Scholar] [CrossRef]
- Toschi, E.; Sgadari, C.; Malavasi, L.; Bacigalupo, I.; Chiozzini, C.; Carlei, D.; Compagnoni, D.; Bellino, S.; Bugarini, R.; Falchi, M.; et al. Human immunodeficiency virus protease inhibitors reduce the growth of human tumors via a proteasome-independent block of angiogenesis and matrix metalloproteinases. Int. J. Cancer 2011, 128, 82–93. [Google Scholar] [CrossRef]
- Pajonk, F.; Himmelsbach, J.; Riess, K.; Sommer, A.; McBride, W.H. The human immunodeficiency virus (HIV)-1 protease inhibitor saquinavir inhibits proteasome function and causes apoptosis and radiosensitization in non-HIV-associated human cancer cells. Cancer Res. 2002, 62, 5230–5235. [Google Scholar]
- Mühl, H.; Paulukat, J.; Höfler, S.; Hellmuth, M.; Franzen, R.; Pfeilschifter, J. The HIV protease inhibitor ritonavir synergizes with butyrate for induction of apoptotic cell death and mediates expression of heme oxygenase-1 in DLD-1 colon carcinoma cells. Br. J. Pharmacol. 2004, 143, 890–898. [Google Scholar] [CrossRef] [Green Version]
- Pati, S.; Pelser, C.B.; Dufraine, J.; Bryant, J.L.; Reitz, M.S., Jr.; Weichold, F.F. Antitumorigenic effects of HIV protease inhibitor ritonavir: Inhibition of Kaposi sarcoma. Blood 2002, 99, 3771–3779. [Google Scholar] [CrossRef] [Green Version]
- Gaedicke, S.; Firat-Geier, E.; Constantiniu, O.; Lucchiari-Hartz, M.; Freudenberg, M.; Galanos, C.; Niedermann, G. Antitumor effect of the human immunodeficiency virus protease inhibitor ritonavir: Induction of tumor-cell apoptosis associated with perturbation of proteasomal proteolysis. Cancer Res. 2002, 62, 6901–6908. [Google Scholar]
- Alburquerque-González, B.; Bernabé-García, Á.; Bernabé-García, M.; Ruiz-Sanz, J.; López-Calderón, F.F.; Gonnelli, L.; Banci, L.; Peña-García, J.; Luque, I.; Nicolás, F.J.; et al. The FDA-Approved Antiviral Raltegravir Inhibits Fascin1-Dependent Invasion of Colorectal Tumor Cells In Vitro and In Vivo. Cancers 2021, 13, 861. [Google Scholar] [CrossRef]
- Onoda, T.; Ono, T.; Dhar, D.K.; Yamanoi, A.; Nagasue, N. Tetracycline analogues (doxycycline and COL-3) induce caspase-dependent and -independent apoptosis in human colon cancer cells. Int. J. Cancer 2006, 118, 1309–1315. [Google Scholar] [CrossRef]
- Onoda, T.; Ono, T.; Dhar, D.K.; Yamanoi, A.; Fujii, T.; Nagasue, N. Doxycycline inhibits cell proliferation and invasive potential: Combination therapy with cyclooxygenase-2 inhibitor in human colorectal cancer cells. J. Lab. Clin. Med. 2004, 143, 207–216. [Google Scholar] [CrossRef]
- Carella, A.M.; Beltrami, G.; Pica, G.; Carella, A.; Catania, G. Clarithromycin potentiates tyrosine kinase inhibitor treatment in patients with resistant chronic myeloid leukemia. Leuk. Lymphoma 2012, 53, 1409–1411. [Google Scholar] [CrossRef]
- Yatsunami, J.; Turuta, N.; Wakamatsu, K.; Hara, N.; Hayashi, S. Clarithromycin is a potent inhibitor of tumor-induced angiogenesis. Res. Exp. Med. 1997, 197, 189–197. [Google Scholar] [CrossRef]
- Petroni, G.; Bagni, G.; Iorio, J.; Duranti, C.; Lottini, T.; Stefanini, M.; Kragol, G.; Becchetti, A.; Arcangeli, A. Clarithromycin inhibits autophagy in colorectal cancer by regulating the hERG1 potassium channel interaction with PI3K. Cell Death Dis. 2020, 11, 161. [Google Scholar] [CrossRef] [Green Version]
- Schafranek, L.; Leclercq, T.M.; White, D.L.; Hughes, T.P. Clarithromycin enhances dasatinib-induced cell death in chronic myeloid leukemia cells, by inhibition of late stage autophagy. Leuk. Lymphoma 2013, 54, 198–201. [Google Scholar] [CrossRef]
- Tanaka, H.; Hino, H.; Moriya, S.; Kazama, H.; Miyazaki, M.; Takano, N.; Hiramoto, M.; Tsukahara, K.; Miyazawa, K. Comparison of autophagy inducibility in various tyrosine kinase inhibitors and their enhanced cytotoxicity via inhibition of autophagy in cancer cells in combined treatment with azithromycin. Biochem. Biophys. Rep. 2020, 22, 100750. [Google Scholar] [CrossRef]
- Toriyama, K.; Takano, N.; Kokuba, H.; Kazama, H.; Moriya, S.; Hiramoto, M.; Abe, S.; Miyazawa, K. Azithromycin enhances the cytotoxicity of DNA-damaging drugs via lysosomal membrane permeabilization in lung cancer cells. Cancer Sci. 2021, 112, 3324–3337. [Google Scholar] [CrossRef]
- Qiao, X.; Wang, X.; Shang, Y.; Li, Y.; Chen, S.Z. Azithromycin enhances anticancer activity of TRAIL by inhibiting autophagy and up-regulating the protein levels of DR4/5 in colon cancer cells in vitro and in vivo. Cancer Commun. 2018, 38, 43. [Google Scholar] [CrossRef] [Green Version]
- Kan, J.Y.; Hsu, Y.L.; Chen, Y.H.; Chen, T.C.; Wang, J.Y.; Kuo, P.L. Gemifloxacin, a fluoroquinolone antimicrobial drug, inhibits migration and invasion of human colon cancer cells. Biomed Res. Int. 2013, 2013, 159786. [Google Scholar] [CrossRef]
- Verma, S.; Das, P.; Kumar, V.L. Chemoprevention by artesunate in a preclinical model of colorectal cancer involves down regulation of β-catenin, suppression of angiogenesis, cellular proliferation and induction of apoptosis. Chem. Biol. Interact. 2017, 278, 84–91. [Google Scholar] [CrossRef]
- Kumar, V.L.; Verma, S.; Das, P. Artesunate suppresses inflammation and oxidative stress in a rat model of colorectal cancer. Drug Dev. Res. 2019, 80, 1089–1097. [Google Scholar] [CrossRef]
- Xu, X.; Wang, J.; Han, K.; Li, S.; Xu, F.; Yang, Y. Antimalarial drug mefloquine inhibits nuclear factor kappa B signaling and induces apoptosis in colorectal cancer cells. Cancer Sci. 2018, 109, 1220–1229. [Google Scholar] [CrossRef] [Green Version]
- Patel, M.M.; Patel, B.M. Repurposing of sodium valproate in colon cancer associated with diabetes mellitus: Role of HDAC inhibition. Eur. J. Pharm. Sci. 2018, 121, 188–199. [Google Scholar] [CrossRef]
- Koh, S.J.; Kim, J.M.; Kim, I.K.; Kim, N.; Jung, H.C.; Song, I.S.; Kim, J.S. Fluoxetine inhibits NF-κB signaling in intestinal epithelial cells and ameliorates experimental colitis and colitis-associated colon cancer in mice. Am. J. Physiol. Gastrointest Liver Physiol. 2011, 301, G9–G19. [Google Scholar] [CrossRef] [Green Version]
- Kannen, V.; Hintzsche, H.; Zanette, D.L.; Silva, W.A.; Garcia, S.B.; Waaga-Gasser, A.M.; Stopper, H. Antiproliferative effects of fluoxetine on colon cancer cells and in a colonic carcinogen mouse model. PLoS ONE 2012, 7, e50043. [Google Scholar] [CrossRef] [Green Version]
- Mussin, N.; Oh, S.C.; Lee, K.W.; Park, M.Y.; Seo, S.; Yi, N.J.; Kim, H.; Yoon, K.C.; Ahn, S.W.; Kim, H.S.; et al. Sirolimus and Metformin Synergistically Inhibits Colon Cancer In Vitro and In Vivo. J. Korean Med. Sci. 2017, 32, 1385–1395. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Lu, J.; Zhang, P.; Xu, Y.; Wang, Z.; Mao, J.H.; Wei, G. Rapamycin inhibits FBXW7 loss-induced epithelial-mesenchymal transition and cancer stem cell-like characteristics in colorectal cancer cells. Biochem. Biophys. Res. Commun. 2013, 434, 352–356. [Google Scholar] [CrossRef] [Green Version]
- He, K.; Zheng, X.; Li, M.; Zhang, L.; Yu, J. mTOR inhibitors induce apoptosis in colon cancer cells via CHOP-dependent DR5 induction on 4E-BP1 dephosphorylation. Oncogene 2016, 35, 148–157. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.; Liu, L.; Chang, E.B.; Wang, J.Y.; Raufman, J.P. Butyrate inhibits pro-proliferative miR-92a by diminishing c-Myc-induced miR-17-92a cluster transcription in human colon cancer cells. Mol. Cancer 2015, 14, 180. [Google Scholar] [CrossRef] [Green Version]
- Hradec, J. Pharmacological therapy for chronic heart failure. Vnitr. Lek. 2018, 64, 853–859. [Google Scholar] [CrossRef]
- Do Vale, G.T.; Ceron, C.S.; Gonzaga, N.A.; Simplicio, J.A.; Padovan, J.C. Three Generations of β-blockers: History, Class Differences and Clinical Applicability. Curr. Hypertens. Rev. 2019, 15, 22–31. [Google Scholar] [CrossRef]
- Engineer, D.R.; Burney, B.O.; Hayes, T.G.; Garcia, J.M. Exposure to ACEI/ARB and β-Blockers Is Associated with Improved Survival and Decreased Tumor Progression and Hospitalizations in Patients with Advanced Colon Cancer. Transl. Oncol. 2013, 6, 539–545. [Google Scholar] [CrossRef] [Green Version]
- Bacchi, S.; Palumbo, P.; Sponta, A.; Coppolino, M.F. Clinical pharmacology of non-steroidal anti-inflammatory drugs: A review. Antiinflamm. Antiallergy Agents Med. Chem. 2012, 11, 52–64. [Google Scholar] [CrossRef]
- Sheng, J.; Sun, H.; Yu, F.B.; Li, B.; Zhang, Y.; Zhu, Y.T. The Role of Cyclooxygenase-2 in Colorectal Cancer. Int. J. Med. Sci. 2020, 17, 1095–1101. [Google Scholar] [CrossRef]
- McAdam, B.F.; Catella-Lawson, F.; Mardini, I.A.; Kapoor, S.; Lawson, J.A.; FitzGerald, G.A. Systemic biosynthesis of prostacyclin by cyclooxygenase (COX)-2: The human pharmacology of a selective inhibitor of COX-2. Proc. Natl. Acad. Sci. USA 1999, 96, 272–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pal, I.; Dey, K.K.; Chaurasia, M.; Parida, S.; Das, S.; Rajesh, Y.; Sharma, K.; Chowdhury, T.; Mandal, M. Cooperative effect of BI-69A11 and celecoxib enhances radiosensitization by modulating DNA damage repair in colon carcinoma. Tumour Biol. 2016, 37, 6389–6402. [Google Scholar] [CrossRef] [PubMed]
- Dulai, P.S.; Singh, S.; Marquez, E.; Khera, R.; Prokop, L.J.; Limburg, P.J.; Gupta, S.; Murad, M.H. Chemoprevention of colorectal cancer in individuals with previous colorectal neoplasia: Systematic review and network meta-analysis. BMJ 2016, 355, i6188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bansal, A.B.; Cassagnol, M. HMG-CoA Reductase Inhibitors. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Poynter, J.N.; Gruber, S.B.; Higgins, P.D.; Almog, R.; Bonner, J.D.; Rennert, H.S.; Low, M.; Greenson, J.K.; Rennert, G. Statins and the risk of colorectal cancer. N. Engl. J. Med. 2005, 352, 2184–2192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buse, J.B.; Wexler, D.J.; Tsapas, A.; Rossing, P.; Mingrone, G.; Mathieu, C.; D’Alessio, D.A.; Davies, M.J. 2019 Update to: Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020, 43, 487–493. [Google Scholar] [CrossRef] [Green Version]
- Berkovic, M.C.; Mikulic, D.; Bilic-Curcic, I.; Mrzljak, A. How far along are we in revealing the connection between metformin and colorectal cancer? World J. Gastroenterol. 2021, 27, 1362–1368. [Google Scholar] [CrossRef]
- Cunha Júnior, A.D.; Bragagnoli, A.C.; Costa, F.O.; Carvalheira, J.B.C. Repurposing metformin for the treatment of gastrointestinal cancer. World J. Gastroenterol. 2021, 27, 1883–1904. [Google Scholar] [CrossRef]
- Pollak, M. Insulin, insulin-like growth factors and neoplasia. Best Pract. Res. Clin. Endocrinol. Metab. 2008, 22, 625–638. [Google Scholar] [CrossRef]
- Pollak, M. The insulin and insulin-like growth factor receptor family in neoplasia: An update. Nat. Rev. Cancer 2012, 12, 159–169. [Google Scholar] [CrossRef]
- Khader, E.I.; Ismail, W.W.; Mhaidat, N.M.; Alqudah, M.A. Effect of metformin on irinotecan-induced cell cycle arrest in colorectal cancer cell lines HCT116 and SW480. Int. J. Health Sci. 2021, 15, 34–41. [Google Scholar]
- Kim, S.H.; Kim, S.C.; Ku, J.L. Metformin increases chemo-sensitivity via gene downregulation encoding DNA replication proteins in 5-Fu resistant colorectal cancer cells. Oncotarget 2017, 8, 56546–56557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha, G.Z.; Dias, M.M.; Ropelle, E.R.; Osório-Costa, F.; Rossato, F.A.; Vercesi, A.E.; Saad, M.J.; Carvalheira, J.B. Metformin amplifies chemotherapy-induced AMPK activation and antitumoral growth. Clin. Cancer Res. 2011, 17, 3993–4005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganesan, K.; Rana, M.B.M.; Sultan, S. Oral Hypoglycemic Medications. In StatPearls, 2021 January ed.; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Kothiwale, S.; Borza, C.M.; Lowe, E.W.; Pozzi, A.; Meiler, J. Discoidin domain receptor 1 (DDR1) kinase as target for structure-based drug discovery. Drug Discov. Today 2015, 20, 255–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okada, J.; Matsumoto, S.; Kaira, K.; Saito, T.; Yamada, E.; Yokoo, H.; Katoh, R.; Kusano, M.; Okada, S.; Yamada, M. Sodium Glucose Cotransporter 2 Inhibition Combined With Cetuximab Significantly Reduced Tumor Size and Carcinoembryonic Antigen Level in Colon Cancer Metastatic to Liver. Clin. Colorectal. Cancer 2018, 17, e45–e48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacey, E. Mode of action of benzimidazoles. Parasitol. Today 1990, 6, 112–115. [Google Scholar] [CrossRef]
- Laudisi, F.; Marônek, M.; Di Grazia, A.; Monteleone, G.; Stolfi, C. Repositioning of Anthelmintic Drugs for the Treatment of Cancers of the Digestive System. Int. J. Mol. Sci. 2020, 21, 4957. [Google Scholar] [CrossRef]
- Nygren, P.; Larsson, R. Drug repositioning from bench to bedside: Tumour remission by the antihelmintic drug mebendazole in refractory metastatic colon cancer. Acta Oncol. 2014, 53, 427–428. [Google Scholar] [CrossRef]
- Chen, W.; Mook, R.A.; Premont, R.T.; Wang, J. Niclosamide: Beyond an antihelminthic drug. Cell. Signal. 2018, 41, 89–96. [Google Scholar] [CrossRef]
- Segditsas, S.; Tomlinson, I. Colorectal cancer and genetic alterations in the Wnt pathway. Oncogene 2006, 25, 7531–7537. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Ren, X.R.; Piao, H.; Zhao, S.; Osada, T.; Premont, R.T.; Mook, R.A.; Morse, M.A.; Lyerly, H.K.; Chen, W. Niclosamide-induced Wnt signaling inhibition in colorectal cancer is mediated by autophagy. Biochem. J. 2019, 476, 535–546. [Google Scholar] [CrossRef]
- Kang, H.E.; Seo, Y.; Yun, J.S.; Song, S.H.; Han, D.; Cho, E.S.; Cho, S.B.; Jeon, Y.; Lee, H.; Kim, H.S.; et al. Metformin and Niclosamide Synergistically Suppress Wnt and YAP in APC-Mutated Colorectal Cancer. Cancers 2021, 13, 3437. [Google Scholar] [CrossRef] [PubMed]
- Wassner, C.; Bradley, N.; Lee, Y. A Review and Clinical Understanding of Tenofovir: Tenofovir Disoproxil Fumarate versus Tenofovir Alafenamide. J. Int. Assoc. Provid. AIDS Care 2020, 19, 2325958220919231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hukezalie, K.R.; Thumati, N.R.; Côté, H.C.; Wong, J.M. In vitro and ex vivo inhibition of human telomerase by anti-HIV nucleoside reverse transcriptase inhibitors (NRTIs) but not by non-NRTIs. PLoS ONE 2012, 7, e47505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jafri, M.A.; Ansari, S.A.; Alqahtani, M.H.; Shay, J.W. Roles of telomeres and telomerase in cancer, and advances in telomerase-targeted therapies. Genome Med. 2016, 8, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanford, S.L.; Welfer, G.A.; Freudenthal, B.D.; Opresko, P.L. Mechanisms of telomerase inhibition by oxidized and therapeutic dNTPs. Nat. Commun. 2020, 11, 5288. [Google Scholar] [CrossRef]
- Kinloch-De Loës, S.; Hirschel, B.J.; Hoen, B.; Cooper, D.A.; Tindall, B.; Carr, A.; Saurat, J.H.; Clumeck, N.; Lazzarin, A.; Mathiesen, L. A controlled trial of zidovudine in primary human immunodeficiency virus infection. N. Engl. J. Med. 1995, 333, 408–413. [Google Scholar] [CrossRef]
- Flexner, C. HIV-protease inhibitors. N. Engl. J. Med. 1998, 338, 1281–1292. [Google Scholar] [CrossRef]
- Blanco, J.L.; Whitlock, G.; Milinkovic, A.; Moyle, G. HIV integrase inhibitors: A new era in the treatment of HIV. Expert Opin. Pharmacother. 2015, 16, 1313–1324. [Google Scholar] [CrossRef]
- Shi, S.; Zheng, H.C.; Zhang, Z.G. Roles of Fascin mRNA expression in colorectal cancer: Meta-analysis and bioinformatics analysis. Mol. Clin. Oncol. 2020, 13, 119–128. [Google Scholar] [CrossRef]
- Tampakis, A.; Tampaki, E.C.; Nonni, A.; Kostakis, I.D.; Posabella, A.; Kontzoglou, K.; von Flue, M.; Felekouras, E.; Kouraklis, G.; Nikiteas, N. High fascin-1 expression in colorectal cancer identifies patients at high risk for early disease recurrence and associated mortality. BMC Cancer 2021, 21, 153. [Google Scholar] [CrossRef]
- Cunha, B.A.; Sibley, C.M.; Ristuccia, A.M. Doxycycline. Ther. Drug Monit. 1982, 4, 115–135. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.J.; Razavian, M.; Kim, H.Y.; Ye, Y.; Golestani, R.; Toczek, J.; Zhang, J.; Sadeghi, M.M. Matrix metalloproteinase inhibitor, doxycycline and progression of calcific aortic valve disease in hyperlipidemic mice. Sci. Rep. 2016, 6, 32659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komatsu, S.; Moriya, S.; Che, X.F.; Yokoyama, T.; Kohno, N.; Miyazawa, K. Combined treatment with SAHA, bortezomib, and clarithromycin for concomitant targeting of aggresome formation and intracellular proteolytic pathways enhances ER stress-mediated cell death in breast cancer cells. Biochem. Biophys. Res. Commun. 2013, 437, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Kikukawa, Y.; Takeya, M.; Mitsuya, H.; Hata, H. Clarithromycin attenuates autophagy in myeloma cells. Int. J. Oncol. 2010, 37, 815–820. [Google Scholar] [PubMed]
- Mokarram, P.; Albokashy, M.; Zarghooni, M.; Moosavi, M.A.; Sepehri, Z.; Chen, Q.M.; Hudecki, A.; Sargazi, A.; Alizadeh, J.; Moghadam, A.R.; et al. New frontiers in the treatment of colorectal cancer: Autophagy and the unfolded protein response as promising targets. Autophagy 2017, 13, 781–819. [Google Scholar] [CrossRef]
- Burada, F.; Nicoli, E.R.; Ciurea, M.E.; Uscatu, D.C.; Ioana, M.; Gheonea, D.I. Autophagy in colorectal cancer: An important switch from physiology to pathology. World J. Gastrointest. Oncol. 2015, 7, 271–284. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Fontaine, S.; Adam, H.; Schurek, K.; Mayer, M.; Noreddin, A.M.; Gin, A.S.; Rubinstein, E.; Hoban, D.J. A Review of New Fluoroquinolones: Focus on their Use in Respiratory Tract Infections. Treat. Respir. Med. 2006, 5, 437–465. [Google Scholar] [CrossRef]
- Barradell, L.B.; Fitton, A. Artesunate. A review of its pharmacology and therapeutic efficacy in the treatment of malaria. Drugs 1995, 50, 714–741. [Google Scholar] [CrossRef]
- Efferth, T.; Sauerbrey, A.; Olbrich, A.; Gebhart, E.; Rauch, P.; Weber, H.O.; Hengstler, J.G.; Halatsch, M.E.; Volm, M.; Tew, K.D.; et al. Molecular modes of action of artesunate in tumor cell lines. Mol. Pharmacol. 2003, 64, 382–394. [Google Scholar] [CrossRef] [Green Version]
- Bai, L.; Xu, Z.; Huang, C.; Sui, Y.; Guan, X.; Shi, L. Psychotropic medication utilisation in adult cancer patients in China: A cross-sectional study based on national health insurance database. Lancet Reg. Health West. Pac. 2020, 5, 100060. [Google Scholar] [CrossRef]
- Ng, C.G.; Boks, M.P.; Smeets, H.M.; Zainal, N.Z.; de Wit, N.J. Prescription patterns for psychotropic drugs in cancer patients; a large population study in the Netherlands. Psychooncology 2013, 22, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Friedmann, I.; Atmaca, A.; Chow, K.U.; Jager, E.; Weidmann, E. Synergistic effects of valproic acid and mitomycin C in adenocarcinoma cell lines and fresh tumor cells of patients with colon cancer. J. Chemother. 2006, 18, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Mologni, L.; Cleris, L.; Magistroni, V.; Piazza, R.; Boschelli, F.; Formelli, F.; Gambacorti-Passerini, C. Valproic acid enhances bosutinib cytotoxicity in colon cancer cells. Int. J. Cancer 2009, 124, 1990–1996. [Google Scholar] [CrossRef] [PubMed]
- Göttlicher, M.; Minucci, S.; Zhu, P.; Krämer, O.H.; Schimpf, A.; Giavara, S.; Sleeman, J.P.; Lo Coco, F.; Nervi, C.; Pelicci, P.G.; et al. Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J. 2001, 20, 6969–6978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benfield, P.; Heel, R.C.; Lewis, S.P. Fluoxetine. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in depressive illness. Drugs 1986, 32, 481–508. [Google Scholar] [CrossRef] [PubMed]
- Sehgal, S.N. Sirolimus: Its discovery, biological properties, and mechanism of action. Transplant. Proc. 2003, 35, 7S–14S. [Google Scholar] [CrossRef]
- Hernández-Lemus, E.; Martínez-García, M. Pathway-Based Drug-Repurposing Schemes in Cancer: The Role of Translational Bioinformatics. Front. Oncol. 2020, 10, 605680. [Google Scholar] [CrossRef]
- Fu, L.; Jin, W.; Zhang, J.; Zhu, L.; Lu, J.; Zhen, Y.; Zhang, L.; Ouyang, L.; Liu, B.; Yu, H. Repurposing non-oncology small-molecule drugs to improve cancer therapy: Current situation and future directions. Acta Pharm. Sin. B 2021, 12, 532–557. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef]
- Wu, P.; Feng, Q.; Kerchberger, V.E.; Nelson, S.D.; Chen, Q.; Li, B.; Edwards, T.L.; Cox, N.J.; Phillips, E.J.; Stein, C.M.; et al. Integrating gene expression and clinical data to identify drug repurposing candidates for hyperlipidemia and hypertension. Nat. Commun. 2022, 13, 46. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, C.; Fu, J.; Lilley, B.; Berlinicke, C.; Hansen, B.; Ding, D.; Wang, G.; Wang, T.; Shou, D.; et al. Large-scale phenotypic drug screen identifies neuroprotectants in zebrafish and mouse models of retinitis pigmentosa. eLife 2021, 10, e57245. [Google Scholar] [CrossRef] [PubMed]
- Oprea, T.I.; Bauman, J.E.; Bologa, C.G.; Buranda, T.; Chigaev, A.; Edwards, B.S.; Jarvik, J.W.; Gresham, H.D.; Haynes, M.K.; Hjelle, B.; et al. Drug Repurposing from an Academic Perspective. Drug Discov. Today Ther. Strateg. 2011, 8, 61–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neuberger, A.; Oraiopoulos, N.; Drakeman, D.L. Renovation as innovation: Is repurposing the future of drug discovery research? Drug Discov. Today 2019, 24, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.A. Lack of Effectiveness of Repurposed Drugs for COVID-19 Treatment. Front. Immunol. 2021, 12, 635371. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, M.I.; de Wolf, E.; Jawad, M.J.; Richardson, A. The poor design of clinical trials of statins in oncology may explain their failure—Lessons for drug repurposing. Cancer Treat Rev. 2018, 69, 84–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Reference | Drug | Original Indication | Possible Mode(s) of Action | Effect(s) |
|---|---|---|---|---|
| [38,39] | ACEIs/ARBs | Hypertension | Decreased chronic inflammation and oxidative stress | Reduced risk of adenomatous colon polyps |
| [40] | Nebivolol | Hypertension and other indications | Inhibition of mitochondrial respiration by decreasing the activity of Complex I of the respiratory chain | Suppressed the growth of colon cancer cells |
| [41,42,43,44] | Aspirin | Antiplatelet | Inhibition of COX-2, c-MYC transcription factor, and the antiplatelet mechanism of action | Decreased cancer metastasis and immune evasion |
| [45,46] | Celecoxib | Anti-inflammatory | Effect on p53 by regulating the expression of p21 and CyclinD1 in a COX-2-independent manner Upregulation of BCCIP Increased radiosensitivity in HCT116 cell line | Decreased incidence of adenomatous polyps. |
| [47,48] | Lovastatin | Antilipidemic | Inhibition of MACC1 | Restricted cancer progression and metastasis formation |
| [49,50,51,52,53] | Metformin | Antihyperglycemic | Inhibition of mTOR Modulation of oxidative stress and nuclear factor-κB inflammatory responses | Apoptosis in CRC cell lines |
| [54,55] | Dapagliflozin | Antihyperglycemic | Effect on cellular interaction with Collagen types I and IV Increased Erk phosphorylation | Decreased adhesion and proliferation of colon cancer cells |
| [56,57] | Mebendazole | Anti-helminthic | Inhibition of MYC | Cytotoxic activity against different colon cancer cell lines |
| [58,59] | Niclosamide | Anti-helminthic | Downregulation of the Wnt/β-catenin cascade | Decreased proliferation in multiple human CRC cell lines |
| [60] | Tenofovir | Anti-retroviral (anti-HIV drug) | Decreased Bcl-2 and cyclin D1 expression | Inhibition of proliferation, oxidative stress, and inflammation |
| [61,62] | Zidovudine | Anti-retroviral (anti-HIV drug) | Increased expression of the p53-Puma/Bax/Noxa pathways Activation of the p53-p21 pathway | Apoptosis Cell cycle arrest |
| [63] | Efavirenz | Anti-retroviral (anti-HIV drug) | Activation of the phosphorylation of p53 | Cytotoxic activity against different colon cancer cell lines |
| [64] | Indinavir | Anti-retroviral (anti-HIV drug) | Proteasome-independent block of angiogenesis and matrix metalloproteinases | Suppressed growth |
| [64,65] | Saquinavir | Anti-retroviral (anti-HIV drug) | Proteasome-independent block of angiogenesis and matrix metalloproteinases Inhibition of proteolytic degradation and accumulation of p21 | Apoptosis Suppressed growth |
| [66,67,68] | Ritonavir | Anti-retroviral (anti-HIV drug) | Inhibition proteolytic degradation and accumulation of p21 Decreased production of TNF-α, IL-6, IL-8, and VEGF Increased expression of heme oxygenase-1 | Apoptosis Suppressed angiogenesis |
| [69] | Raltegravir | Anti-retroviral (anti-HIV drug) | Blockage of fascin-1 | Suppressed invasion |
| [70,71] | Doxycycline | Antibiotic | Inhibition of matrix metalloproteinases Activation of caspase-3, -8, and -9 Release of cytochrome c and Bax translocation | Apoptosis Suppressed proliferation and invasive potential |
| [72,73,74,75] | Clarithromycin | Antibiotic | Inhibition of autophagy by targeting hERG1 | Suppressed angiogenesis Suppressed growth of colon cancer cells |
| [76,77,78] | Azithromycin | Antibiotic | Inhibition of autophagy by upregulating p62 and LC-3B | Apoptosis |
| [79] | Gemifloxacin | Antibiotic | Inhibition of NF-κB activation Inhibition of TNF-α, IL-6, IL-8, and VEGF | Suppressed cell migration and invasion |
| [80,81] | Artesunate | Antimalarial | Downregulation of β-catenin | Apoptosis Cytotoxicity |
| [82] | Mefloquine | Antimalarial | Inhibition of NF-κB activation | Apoptosis Growth arrest |
| [83] | Valproate | Antipsychotic | Histone hyperacetylation Relief of HDAC-mediated transcriptional repression | Reduced viability Enhanced cytotoxicity |
| [84,85] | Fluoxetine | Antidepressant | Inhibition of NF-κB activation and IKK phosphorylation Cell-cycle arrest at G0/G1 Enhanced p27 expression Reduced VEGF expression | Suppressed colitis-associated tumorigenesis Suppressed dysplasia and angiogenesis |
| [86,87,88] | Sirolimus | Prevention of kidney transplant rejection | CHOP-dependent DR5 induction on 4E-BP1 dephosphorylation Suppressed FBXW7 loss-driven EMT | Apoptosis Decreased angiogenesis Suppressed proliferation and invasion of colon cancer cells |
| [89] | Butyrate | Probiotic | Inhibition of miR-92a | Suppressed proliferation of colon cancer cells |
| Clinical Trial Number | Phase | Status | Estimated Completion Date | Intervention/Treatment | Patient Population | Patients Enrolled | Primary Outcome Measures | Secondary Outcome Measures |
|---|---|---|---|---|---|---|---|---|
| NCT02467582 | 3 | Active, not recruiting | June 2029 | Aspirin | Stages II and III PIK3CA-mutated CRC previously treated with surgery | 185 | DFS after 6 years | Time to recurrenceOS Cancer-specific survival Adverse events |
| NCT02301286 | 3 | Recruiting | September 2022 | Aspirin | Stages II and III CRC | 1588 | OS | DFS TTF |
| NCT03464305 | 3 | Recruiting | December 2026 | Aspirin | Stages II and III CRC | 400 | 5-year OS | DFS TTF |
| NCT02945033 | 3 | Recruiting | July 2024 | Aspirin | PI3K-mutated CRC | 246 | Recurrence or second CRC or death, whichever occurs first | 5-year OS Adverse events |
| NCT00565708 | 3 | Active, not recruiting | June 2026 | Aspirin | Dukes C and high-risk Dukes B CRCs | 1587 | DFS | OS |
| NCT03026140 | 2 | Recruiting | January 2022 | Nivolumab + Ipilimumab with or without Celecoxib | Stages I to III CRC | 60 | Incidence of adverse events | Immune activating capacity of immunotherapyRelapse-free survival |
| NCT03925662 | 3 | Recruiting | December 2028 | FOLFOX + bevacizumab with or without mebendazole | Stage IV CRC | 40 | ORR | - |
| NCT03359681 | 2 | Recruiting | January 2022 | Metformin | CRC | 48 | Ki67 expression on tumor samples | Cleaved Caspase-3 expression Immunoscore Immunological changes in blood samples In vitro cell growth |
| NCT04873895 | 1 | Recruiting | November 2023 | Axitinib + hydroxychloroquine | Liver-dominant metastatic CRC | 25 | Serious adverse events | ORR in setting of liver metastasis PFS OS |
| NCT03919292 | 1/2 | Recruiting | January 2024 | Neratinib + valproate | Advanced solid tumors including CRC | 113 | Recommended phase 2 dose | Adverse events Antitumor effects PFS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Zarif, T.; Yibirin, M.; De Oliveira-Gomes, D.; Machaalani, M.; Nawfal, R.; Bittar, G.; Bahmad, H.F.; Bitar, N. Overcoming Therapy Resistance in Colon Cancer by Drug Repurposing. Cancers 2022, 14, 2105. https://doi.org/10.3390/cancers14092105
El Zarif T, Yibirin M, De Oliveira-Gomes D, Machaalani M, Nawfal R, Bittar G, Bahmad HF, Bitar N. Overcoming Therapy Resistance in Colon Cancer by Drug Repurposing. Cancers. 2022; 14(9):2105. https://doi.org/10.3390/cancers14092105
Chicago/Turabian StyleEl Zarif, Talal, Marcel Yibirin, Diana De Oliveira-Gomes, Marc Machaalani, Rashad Nawfal, Gianfranco Bittar, Hisham F. Bahmad, and Nizar Bitar. 2022. "Overcoming Therapy Resistance in Colon Cancer by Drug Repurposing" Cancers 14, no. 9: 2105. https://doi.org/10.3390/cancers14092105