Vitamin D Metabolites in Nonmetastatic High-Risk Prostate Cancer Patients with and without Zoledronic Acid Treatment after Prostatectomy
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
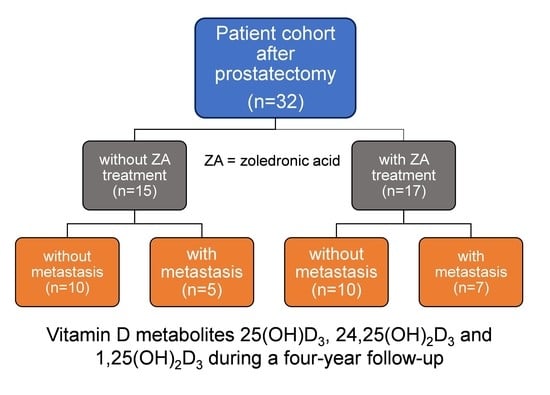
2.1. Patients and Samples
2.2. Analytics for Vitamin D Metabolites
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics and Study Design
3.2. Vitamin D3 Metabolites in the Total Study Cohort and Dependency on the Season of the Start of the Study
3.3. Vitamin D3 Metabolites in Relation to the ZA Treatment
3.4. Vitamin D3 Metabolites in Relation to the Development of Bone Metastasis during the Study
3.5. Correlations between Vitamin D3 Metabolites
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giustina, A.; Bouillon, R.; Binkley, N.; Sempos, C.; Adler, R.A.; Bollerslev, J.; Dawson-Hughes, B.; Ebeling, P.R.; Feldman, D.; Heijboer, A.; et al. Controversies in vitamin D: A statement from the Third International Conference. JBMR Plus 2020, 4, e10417. [Google Scholar] [CrossRef] [PubMed]
- Giustina, A.; Adler, R.A.; Binkley, N.; Bouillon, R.; Ebeling, P.R.; Lazaretti-Castro, M.; Marcocci, C.; Rizzoli, R.; Sempos, C.T.; Bilezikian, J.P. Controversies in vitamin D: Summary Statement from an International Conference. J. Clin. Endocrinol. Metab. 2019, 104, 234–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giustina, A.; Adler, R.A.; Binkley, N.; Bollerslev, J.; Bouillon, R.; Dawson-Hughes, B.; Ebeling, P.R.; Feldman, D.; Formenti, A.M.; Lazaretti-Castro, M.; et al. Consensus statement from 2(nd) International Conference on Controversies in Vitamin D. Rev. Endocr. Metab. Disord. 2020, 21, 89–116. [Google Scholar] [CrossRef] [Green Version]
- Bouillon, R.; Marcocci, C.; Carmeliet, G.; Bikle, D.; White, J.H.; Dawson-Hughes, B.; Lips, P.; Munns, C.F.; Lazaretti-Castro, M.; Giustina, A.; et al. Skeletal and extraskeletal actions of vitamin D: Current evidence and outstanding questions. Endocr. Rev. 2019, 40, 1109–1151. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Johnson, C.S.; Trump, D.L. Mechanistic insights of vitamin D anticancer effects. Vitam. Horm. 2016, 100, 395–431. [Google Scholar] [CrossRef]
- Bilani, N.; Elson, L.; Szuchan, C.; Elimimian, E.; Saleh, M.; Nahleh, Z. Newly-identified pathways relating vitamin D to carcinogenesis: A Review. In Vivo 2021, 35, 1345–1354. [Google Scholar] [CrossRef]
- Jeon, S.M.; Shin, E.A. Exploring vitamin D metabolism and function in cancer. Exp. Mol. Med. 2018, 50, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Amrein, K.; Scherkl, M.; Hoffmann, M.; Neuwersch-Sommeregger, S.; Kostenberger, M.; Tmava Berisha, A.; Martucci, G.; Pilz, S.; Malle, O. Vitamin D deficiency 2.0: An update on the current status worldwide. Eur. J. Clin. Nutr. 2020, 74, 1498–1513. [Google Scholar] [CrossRef]
- Ahn, J.; Park, S.; Zuniga, B.; Bera, A.; Song, C.S.; Chatterjee, B. Vitamin D in prostate cancer. Vitam. Horm. 2016, 100, 321–355. [Google Scholar] [CrossRef] [PubMed]
- Trump, D.L.; Aragon-Ching, J.B. Vitamin D in prostate cancer. Asian J. Androl. 2018, 20, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Capiod, T.; Barry Delongchamps, N.; Pigat, N.; Souberbielle, J.C.; Goffin, V. Do dietary calcium and vitamin D matter in men with prostate cancer? Nat. Rev. Urol. 2018, 15, 453–461. [Google Scholar] [CrossRef]
- Xu, Y.; Shao, X.; Yao, Y.; Xu, L.; Chang, L.; Jiang, Z.; Lin, Z. Positive association between circulating 25-hydroxyvitamin D levels and prostate cancer risk: New findings from an updated meta-analysis. J. Cancer Res. Clin. Oncol. 2014, 140, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Shui, I.M.; Mucci, L.A.; Kraft, P.; Tamimi, R.M.; Lindstrom, S.; Penney, K.L.; Nimptsch, K.; Hollis, B.W.; Dupre, N.; Platz, E.A.; et al. Vitamin D-related genetic variation, plasma vitamin D, and risk of lethal prostate cancer: A prospective nested case-control study. J. Natl. Cancer Inst. 2012, 104, 690–699. [Google Scholar] [CrossRef] [Green Version]
- Fang, F.; Kasperzyk, J.L.; Shui, I.; Hendrickson, W.; Hollis, B.W.; Fall, K.; Ma, J.; Gaziano, J.M.; Stampfer, M.J.; Mucci, L.A.; et al. Prediagnostic plasma vitamin D metabolites and mortality among patients with prostate cancer. PLoS ONE 2011, 6, e18625. [Google Scholar] [CrossRef] [Green Version]
- Brandstedt, J.; Almquist, M.; Manjer, J.; Malm, J. Vitamin D, PTH, and calcium in relation to survival following prostate cancer. Cancer Causes Control 2016, 27, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.Y.; Yao, Q.; Zhuo, Z.; Ma, Z.; Chen, G. Circulating vitamin D level and mortality in prostate cancer patients: A dose-response meta-analysis. Endocr. Connect. 2018, 7, R294–R303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahvazi, S.; Soltani, S.; Ahmadi, S.M.; de Souza, R.J.; Salehi-Abargouei, A. The effect of vitamin D supplementation on prostate cancer: A systematic review and meta-Analysis of clinical trials. Horm. Metab. Res. 2019, 51, 11–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ordonez-Mena, J.M.; Schottker, B.; Fedirko, V.; Jenab, M.; Olsen, A.; Halkjaer, J.; Kampman, E.; de Groot, L.; Jansen, E.; Bueno-de-Mesquita, H.B.; et al. Pre-diagnostic vitamin D concentrations and cancer risks in older individuals: An analysis of cohorts participating in the CHANCES consortium. Eur. J. Epidemiol. 2016, 31, 311–323. [Google Scholar] [CrossRef]
- Gilbert, R.; Metcalfe, C.; Fraser, W.D.; Donovan, J.; Hamdy, F.; Neal, D.E.; Lane, J.A.; Martin, R.M. Associations of circulating 25-hydroxyvitamin D with prostate cancer diagnosis, stage and grade. Int. J. Cancer 2012, 131, 1187–1196. [Google Scholar] [CrossRef] [Green Version]
- Nair-Shalliker, V.; Bang, A.; Egger, S.; Clements, M.; Gardiner, R.A.; Kricker, A.; Seibel, M.J.; Chambers, S.K.; Kimlin, M.G.; Armstrong, B.K.; et al. Post-treatment levels of plasma 25- and 1,25-dihydroxy vitamin D and mortality in men with aggressive prostate cancer. Sci. Rep. 2020, 10, 7736. [Google Scholar] [CrossRef]
- Stroomberg, H.V.; Vojdeman, F.J.; Madsen, C.M.; Helgstrand, J.T.; Schwarz, P.; Heegaard, A.M.; Olsen, A.; Tjonneland, A.; Struer Lind, B.; Brasso, K.; et al. Vitamin D levels and the risk of prostate cancer and prostate cancer mortality. Acta Oncol. 2021, 60, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Stephan, C.; Lein, M.; Matalon, J.; Kilic, E.; Zhao, Z.; Busch, J.; Jung, K. Serum vitamin D is not helpful for predicting prostate cancer aggressiveness compared with the Prostate Health Index. J. Urol. 2016, 196, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Denham, J.W.; Nowitz, M.; Joseph, D.; Duchesne, G.; Spry, N.A.; Lamb, D.S.; Matthews, J.; Turner, S.; Atkinson, C.; Tai, K.H.; et al. Impact of androgen suppression and zoledronic acid on bone mineral density and fractures in the Trans-Tasman Radiation Oncology Group (TROG) 03.04 Randomised Androgen Deprivation and Radiotherapy (RADAR) randomized controlled trial for locally advanced prostate cancer. BJU Int. 2014, 114, 344–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saad, F.; Gleason, D.M.; Murray, R.; Tchekmedyian, S.; Venner, P.; Lacombe, L.; Chin, J.L.; Vinholes, J.J.; Goas, J.A.; Chen, B.; et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J. Natl. Cancer Inst. 2002, 94, 1458–1468. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.V.; Fong, E.M.; Singer, F.R.; Guenette, R.S. Bisphosphonate treatment inhibits the growth of prostate cancer cells. Cancer Res. 2001, 61, 2602–2608. [Google Scholar] [PubMed]
- Wood, J.; Bonjean, K.; Ruetz, S.; Bellahcene, A.; Devy, L.; Foidart, J.M.; Castronovo, V.; Green, J.R. Novel antiangiogenic effects of the bisphosphonate compound zoledronic acid. J. Pharmacol. Exp. Ther. 2002, 302, 1055–1061. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.W.; Huo, D.; Demers, L.M.; Beer, T.M.; Lacerna, L.V. Zoledronic acid initiated during the first year of androgen deprivation therapy increases bone mineral density in patients with prostate cancer. J. Urol. 2006, 176, 972–978. [Google Scholar] [CrossRef]
- Lowrance, W.T.; Breau, R.H.; Chou, R.; Chapin, B.F.; Crispino, T.; Dreicer, R.; Jarrard, D.F.; Kibel, A.S.; Morgan, T.M.; Morgans, A.K.; et al. Advanced Prostate Cancer: AUA/ASTRO/SUO Guideline PART II. J. Urol. 2021, 205, 22–29. [Google Scholar] [CrossRef]
- Mottet, N.; Cornford, P.; van den Bergh, R.C.N.; Briers, E.; De Santis, M.; Gillessen, S.; Grummet, J.; Henry, A.M.; van der Kwast, T.H.; Lam, T.B.; et al. EAU Guidelines. Edn. Presented at the EAU Annual Congress Milan. 2021. Available online: http://uroweb.org/guidelines/compilations-of-all-guidelines/ (accessed on 6 February 2022).
- Prostate Cancer: Diagnosis and Management [A] Evidence Review for Bisphosphonates. NICE Guideline NG131, Published by National Institute for Health and Care Excellence, May 2019, Last Updated December 2021. Available online: http://www.nice.org.uk/guidance/ng131 (accessed on 6 February 2022).
- Saylor, P.J.; Rumble, R.B.; Tagawa, S.; Eastham, J.A.; Finelli, A.; Reddy, P.S.; Kungel, T.M.; Nissenberg, M.G.; Michalski, J.M. Bone health and bone-targeted therapies for prostate cancer: ASCO Endorsement of a Cancer Care Ontario Guideline. J. Clin. Oncol. 2020, 38, 1736–1743. [Google Scholar] [CrossRef]
- Bourke, S.; Bolland, M.J.; Grey, A.; Horne, A.M.; Wattie, D.J.; Wong, S.; Gamble, G.D.; Reid, I.R. The impact of dietary calcium intake and vitamin D status on the effects of zoledronate. Osteoporos. Int. 2013, 24, 349–354. [Google Scholar] [CrossRef]
- Mosali, P.; Bernard, L.; Wajed, J.; Mohamed, Z.; Ewang, M.; Moore, A.; Fogelman, I.; Hampson, G. Vitamin D status and parathyroid hormone concentrations influence the skeletal response to zoledronate and denosumab. Calcif. Tissue Int. 2014, 94, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Wirth, M.; Tammela, T.; Cicalese, V.; Gomez Veiga, F.; Delaere, K.; Miller, K.; Tubaro, A.; Schulze, M.; Debruyne, F.; Huland, H.; et al. Prevention of bone metastases in patients with high-risk nonmetastatic prostate cancer treated with zoledronic acid: Efficacy and safety results of the Zometa European Study (ZEUS). Eur. Urol. 2015, 67, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Hyslop, N.P.; White, W.H. Estimating precision using duplicate measurements. J. Air Waste Manag. Assoc. 2009, 59, 1032–1039. [Google Scholar] [CrossRef] [Green Version]
- Peters, R.; Stephan, C.; Jung, K.; Lein, M.; Friedersdorff, F.; Maxeiner, A. Comparison of PHI and PHI density for prostate cancer detection in a large retrospective Caucasian cohort. Urol. Int. 2021, in press. [Google Scholar] [CrossRef]
- Chesney, R.W.; Rosen, J.F.; Hamstra, A.J.; Smith, C.; Mahaffey, K.; DeLuca, H.F. Absence of seasonal variation in serum concentrations of 1,25-dihydroxyvitamin D despite a rise in 25-hydroxyvitamin D in summer. J. Clin. Endocrinol. Metab. 1981, 53, 139–142. [Google Scholar] [CrossRef]
- Li, H.; Stampfer, M.J.; Hollis, J.B.; Mucci, L.A.; Gaziano, J.M.; Hunter, D.; Giovannucci, E.L.; Ma, J. A prospective study of plasma vitamin D metabolites, vitamin D receptor polymorphisms, and prostate cancer. PLoS Med. 2007, 4, e103. [Google Scholar] [CrossRef] [PubMed]
- Aloia, J.; Fazzari, M.; Shieh, A.; Dhaliwal, R.; Mikhail, M.; Hoofnagle, A.N.; Ragolia, L. The vitamin D metabolite ratio (VMR) as a predictor of functional biomarkers of bone health. Clin. Endocrinol. 2017, 86, 674–679. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.C.; Manson, J.E.; Abrams, S.A.; Aloia, J.F.; Brannon, P.M.; Clinton, S.K.; Durazo-Arvizu, R.A.; Gallagher, J.C.; Gallo, R.L.; Jones, G.; et al. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metab. 2011, 96, 53–58. [Google Scholar] [CrossRef]
- Link, H.; Diel, I.; Ohlmann, C.H.; Holtmann, L.; Kerkmann, M.; for the Associations Supportive Care in Oncology (AGSMO), Medical Oncology (AIO), Urological Oncology (AUO), within the German Cancer Society (DKG) and the German Osteooncological Society (DOG). Guideline adherence in bone-targeted treatment of cancer patients with bone metastases in Germany. Support. Care Cancer 2020, 28, 2175–2184. [Google Scholar] [CrossRef] [Green Version]
- Holick, M.F.; Binkley, N.C.; Bischoff-Ferrari, H.A.; Gordon, C.M.; Hanley, D.A.; Heaney, R.P.; Murad, M.H.; Weaver, C.M.; Endocrine, S. Evaluation, treatment, and prevention of vitamin D deficiency: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2011, 96, 1911–1930. [Google Scholar] [CrossRef] [Green Version]
- Sempos, C.T.; Heijboer, A.C.; Bikle, D.D.; Bollerslev, J.; Bouillon, R.; Brannon, P.M.; DeLuca, H.F.; Jones, G.; Munns, C.F.; Bilezikian, J.P.; et al. Vitamin D assays and the definition of hypovitaminosis D: Results from the First International Conference on Controversies in Vitamin D. Br. J. Clin. Pharmacol. 2018, 84, 2194–2207. [Google Scholar] [CrossRef] [PubMed]
- Altieri, B.; Cavalier, E.; Bhattoa, H.P.; Perez-Lopez, F.R.; Lopez-Baena, M.T.; Perez-Roncero, G.R.; Chedraui, P.; Annweiler, C.; Della Casa, S.; Zelzer, S.; et al. Vitamin D testing: Advantages and limits of the current assays. Eur. J. Clin. Nutr. 2020, 74, 231–247. [Google Scholar] [CrossRef] [PubMed]
- Bollerslev, J.; Rejnmark, L.; Marcocci, C.; Shoback, D.M.; Sitges-Serra, A.; van Biesen, W.; Dekkers, O.M.; European Society of, E. European Society of Endocrinology Clinical Guideline: Treatment of chronic hypoparathyroidism in adults. Eur. J. Endocrinol. 2015, 173, G1–G20. [Google Scholar] [CrossRef] [PubMed]
- Hanley, D.A.; Cranney, A.; Jones, G.; Whiting, S.J.; Leslie, W.D.; Guidelines Committee of the Scientific Advisory Council of Osteoporosis Canada. Vitamin D in adult health and disease: A review and guideline statement from Osteoporosis Canada (summary). CMAJ 2010, 182, 1315–1319. [Google Scholar] [CrossRef] [Green Version]
- Francis, R.M.; Aspray, T.J.; Bowring, C.E.; Fraser, W.D.; Gittoes, N.J.; Javaid, M.K.; Macdonald, H.M.; Patel, S.; Selby, P.L.; Tanna, N. National Osteoporosis Society practical clinical guideline on vitamin D and bone health. Maturitas 2015, 80, 119–121. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Dietary reference values for vitamin D. EFSA J. 2016, 14, 4547. [Google Scholar] [CrossRef]
- Pilz, S.; Zittermann, A.; Trummer, C.; Theiler-Schwetz, V.; Lerchbaum, E.; Keppel, M.H.; Grubler, M.R.; Marz, W.; Pandis, M. Vitamin D testing and treatment: A narrative review of current evidence. Endocr. Connect. 2019, 8, R27–R43. [Google Scholar] [CrossRef] [Green Version]
- Jones, G. Pharmacokinetics of vitamin D toxicity. Am. J. Clin. Nutr. 2008, 88, 582S–586S. [Google Scholar] [CrossRef] [Green Version]
- Heaney, R.P.; Davies, K.M.; Chen, T.C.; Holick, M.F.; Barger-Lux, M.J. Human serum 25-hydroxycholecalciferol response to extended oral dosing with cholecalciferol. Am. J. Clin. Nutr. 2003, 77, 204–210. [Google Scholar] [CrossRef] [Green Version]
- Lips, P.; Wiersinga, A.; van Ginkel, F.C.; Jongen, M.J.; Netelenbos, J.C.; Hackeng, W.H.; Delmas, P.D.; van der Vijgh, W.J. The effect of vitamin D supplementation on vitamin D status and parathyroid function in elderly subjects. J. Clin. Endocrinol. Metab. 1988, 67, 644–650. [Google Scholar] [CrossRef]
- Petkovich, M.; Helvig, C.; Epps, T. CYP24A1 regulation in health and disease. In Vitamin D, 3rd ed.; Feldman, D., Pike, J.W., Adams, J.S., Eds.; Academic Press: New York, NY, USA, 2011; Volume 2, pp. 1525–1554. [Google Scholar]
- Rubsamen, D.; Kunze, M.M.; Buderus, V.; Brauss, T.F.; Bajer, M.M.; Brune, B.; Schmid, T. Inflammatory conditions induce IRES-dependent translation of cyp24a1. PLoS ONE 2014, 9, e85314. [Google Scholar] [CrossRef] [Green Version]
- De Paolis, E.; Scaglione, G.L.; De Bonis, M.; Minucci, A.; Capoluongo, E. CYP24A1 and SLC34A1 genetic defects associated with idiopathic infantile hypercalcemia: From genotype to phenotype. Clin. Chem. Lab. Med. 2019, 57, 1650–1667. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.H.; Powe, C.E.; Evans, M.K.; Wenger, J.; Ortiz, G.; Zonderman, A.B.; Suntharalingam, P.; Lucchesi, K.; Powe, N.R.; Karumanchi, S.A.; et al. 24,25-Dihydroxyvitamin d3 and vitamin D status of community-dwelling black and white Americans. Clin. Chem. 2015, 61, 877–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, D.; Hanwell, H.E.; Schnabl, K.; Yazdanpanah, M.; Kimball, S.; Fu, L.; Sidhom, G.; Rousseau, D.; Cole, D.E.; Vieth, R. The ratio of serum 24,25-dihydroxyvitamin D3 to 25-hydroxyvitamin D3 is predictive of 25-hydroxyvitamin D3 response to vitamin D3 supplementation. J. Steroid Biochem. Mol. Biol. 2011, 126, 72–77. [Google Scholar] [CrossRef]
- Cashman, K.D.; Hayes, A.; Galvin, K.; Merkel, J.; Jones, G.; Kaufmann, M.; Hoofnagle, A.N.; Carter, G.D.; Durazo-Arvizu, R.A.; Sempos, C.T. Significance of serum 24,25-dihydroxyvitamin D in the assessment of vitamin D status: A double-edged sword? Clin. Chem. 2015, 61, 636–645. [Google Scholar] [CrossRef] [Green Version]
- Francic, V.; Ursem, S.R.; Dirks, N.F.; Keppel, M.H.; Theiler-Schwetz, V.; Trummer, C.; Pandis, M.; Borzan, V.; Grubler, M.R.; Verheyen, N.D.; et al. The effect of vitamin D supplementation on its metabolism and the vitamin D metabolite ratio. Nutrients 2019, 11, 2539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.K.; Chung, H.J.; Le, H.G.; Na, B.K.; Cho, M.C. Serum 24,25-dihydroxyvitamin D level in general Korean population and its relationship with other vitamin D biomarkers. PLoS ONE 2021, 16, e0246541. [Google Scholar] [CrossRef]
- Geller, J.L.; Hu, B.; Reed, S.; Mirocha, J.; Adams, J.S. Increase in bone mass after correction of vitamin D insufficiency in bisphosphonate-treated patients. Endocr. Pract. 2008, 14, 293–297. [Google Scholar] [CrossRef]
- Carmel, A.S.; Shieh, A.; Bang, H.; Bockman, R.S. The 25(OH)D level needed to maintain a favorable bisphosphonate response is ≥3 ng/mL. Osteoporos. Int. 2012, 23, 2479–2487. [Google Scholar] [CrossRef] [Green Version]
- Ishijima, M.; Sakamoto, Y.; Yamanaka, M.; Tokita, A.; Kitahara, K.; Kaneko, H.; Kurosawa, H. Minimum required vitamin D level for optimal increase in bone mineral density with alendronate treatment in osteoporotic women. Calcif. Tissue Int. 2009, 85, 398–404. [Google Scholar] [CrossRef]
- Nakamura, Y.; Suzuki, T.; Kamimura, M.; Murakami, K.; Ikegami, S.; Uchiyama, S.; Kato, H. Vitamin D and calcium are required at the time of denosumab administration during osteoporosis treatment. Bone Res. 2017, 5, 17021. [Google Scholar] [CrossRef] [Green Version]
- Alibhai, S.M.H.; Zukotynski, K.; Walker-Dilks, C.; Emmenegger, U.; Finelli, A.; Morgan, S.C.; Hotte, S.J.; Tomlinson, G.A.; Winquist, E. Bone health and bone-targeted therapies for nonmetastatic prostate cancer: A systematic review and meta-analysis. Ann. Intern. Med. 2017, 167, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Dirks, N.F.; Martens, F.; Vanderschueren, D.; Billen, J.; Pauwels, S.; Ackermans, M.T.; Endert, E.; Heijer, M.D.; Blankenstein, M.A.; Heijboer, A.C. Determination of human reference values for serum total 1,25-dihydroxyvitamin D using an extensively validated 2D ID-UPLC-MS/MS method. J. Steroid Biochem. Mol. Biol. 2016, 164, 127–133. [Google Scholar] [CrossRef]
- Wagner, D.; Trudel, D.; Van der Kwast, T.; Nonn, L.; Giangreco, A.A.; Li, D.; Dias, A.; Cardoza, M.; Laszlo, S.; Hersey, K.; et al. Randomized clinical trial of vitamin D3 doses on prostatic vitamin D metabolite levels and Ki67 labeling in prostate cancer patients. J. Clin. Endocrinol. Metab. 2013, 98, 1498–1507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richards, Z.; Batai, K.; Farhat, R.; Shah, E.; Makowski, A.; Gann, P.H.; Kittles, R.; Nonn, L. Prostatic compensation of the vitamin D axis in African American men. JCI Insight 2017, 2, e91054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuckey, R.C.; Cheng, C.Y.S.; Slominski, A.T. The serum vitamin D metabolome: What we know and what is still to discover. J. Steroid Biochem. Mol. Biol. 2019, 186, 4–21. [Google Scholar] [CrossRef]
- Lips, P. Relative value of 25(OH)D and 1,25(OH)2D measurements. J. Bone Miner. Res. 2007, 22, 1668–1671. [Google Scholar] [CrossRef] [PubMed]
- Porojnicu, A.; Robsahm, T.E.; Berg, J.P.; Moan, J. Season of diagnosis is a predictor of cancer survival. Sun-induced vitamin D may be involved: A possible role of sun-induced Vitamin D. J. Steroid Biochem. Mol. Biol. 2007, 103, 675–678. [Google Scholar] [CrossRef]
- Bouillon, R.A.; Auwerx, J.H.; Lissens, W.D.; Pelemans, W.K. Vitamin D status in the elderly: Seasonal substrate deficiency causes 1,25-dihydroxycholecalciferol deficiency. Am. J. Clin. Nutr. 1987, 45, 755–763. [Google Scholar] [CrossRef]
- Hsu, S.; Prince, D.K.; Williams, K.; Allen, N.B.; Burke, G.L.; Hoofnagle, A.N.; Li, X.; Liu, K.J.; McClelland, R.L.; Michos, E.D.; et al. Clinical and biomarker modifiers of vitamin D treatment response: The multi-ethnic study of atherosclerosis. Am. J. Clin. Nutr. 2021, 115, 914–924. [Google Scholar] [CrossRef]
- Shah, I.; Petroczi, A.; Naughton, D.P. Exploring the role of vitamin D in type 1 diabetes, rheumatoid arthritis, and Alzheimer disease: New insights from accurate analysis of 10 forms. J. Clin. Endocrinol. Metab. 2014, 99, 808–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, J.C.Y.; Jackson, S.; Walsh, N.P.; Greeves, J.; Fraser, W.D.; Bioanalytical Facility Team. The dynamic relationships between the active and catabolic vitamin D metabolites, their ratios, and associations with PTH. Sci. Rep. 2019, 9, 6974. [Google Scholar] [CrossRef] [PubMed]
- Dirks, N.F.; Ackermans, M.T.; Lips, P.; de Jongh, R.T.; Vervloet, M.G.; de Jonge, R.; Heijboer, A.C. The when, what & how of measuring vitamin D metabolism in clinical medicine. Nutrients 2018, 10, 482. [Google Scholar] [CrossRef] [Green Version]
- Corder, E.H.; Guess, H.A.; Hulka, B.S.; Friedman, G.D.; Sadler, M.; Vollmer, R.T.; Lobaugh, B.; Drezner, M.K.; Vogelman, J.H.; Orentreich, N. Vitamin D and prostate cancer: A prediagnostic study with stored sera. Cancer Epidemiol. Biomark. Prev. 1993, 2, 467–472. [Google Scholar] [PubMed]
- Rosenberg, A.; Nettey, O.S.; Gogana, P.; Sheikh, U.; Macias, V.; Kajdacsy-Balla, A.; Sharifi, R.; Kittles, R.A.; Murphy, A.B. Physiologic serum 1,25 dihydroxyvitamin D is inversely associated with prostatic Ki67 staining in a diverse sample of radical prostatectomy patients. Cancer Causes Control 2019, 30, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.; Krishnan, A.V.; Feldman, D. Molecular mechanisms mediating the anti-proliferative effects of vitamin D in prostate cancer. J. Steroid Biochem. Mol. Biol. 2005, 97, 31–36. [Google Scholar] [CrossRef]
- Sung, V.; Feldman, D. 1,25-Dihydroxyvitamin D3 decreases human prostate cancer cell adhesion and migration. Mol. Cell. Endocrinol. 2000, 164, 133–143. [Google Scholar] [CrossRef]
- Luo, W.; Yu, W.D.; Ma, Y.; Chernov, M.; Trump, D.L.; Johnson, C.S. Inhibition of protein kinase CK2 reduces Cyp24a1 expression and enhances 1,25-dihydroxyvitamin D3 antitumor activity in human prostate cancer cells. Cancer Res. 2013, 73, 2289–2297. [Google Scholar] [CrossRef] [Green Version]
- Swami, S.; Krishnan, A.V.; Wang, J.Y.; Jensen, K.; Horst, R.; Albertelli, M.A.; Feldman, D. Dietary vitamin D3 and 1,25-dihydroxyvitamin D3 (calcitriol) exhibit equivalent anticancer activity in mouse xenograft models of breast and prostate cancer. Endocrinology 2012, 153, 2576–2587. [Google Scholar] [CrossRef] [Green Version]
- Bao, B.Y.; Yeh, S.D.; Lee, Y.F. 1alpha,25-dihydroxyvitamin D3 inhibits prostate cancer cell invasion via modulation of selective proteases. Carcinogenesis 2006, 27, 32–42. [Google Scholar] [CrossRef]
- Bao, B.Y.; Yao, J.; Lee, Y.F. 1alpha,25-dihydroxyvitamin D3 suppresses interleukin-8-mediated prostate cancer cell angiogenesis. Carcinogenesis 2006, 27, 1883–1893. [Google Scholar] [CrossRef] [PubMed]
- Ben-Eltriki, M.; Deb, S.; Guns, E.S.T. 1alpha,25-Dihydroxyvitamin D3 synergistically enhances anticancer effects of ginsenoside Rh2 in human prostate cancer cells. J. Steroid Biochem. Mol. Biol. 2021, 209, 105828. [Google Scholar] [CrossRef]
- Hershberger, P.A.; Yu, W.D.; Modzelewski, R.A.; Rueger, R.M.; Johnson, C.S.; Trump, D.L. Calcitriol (1,25-dihydroxycholecalciferol) enhances paclitaxel antitumor activity in vitro and in vivo and accelerates paclitaxel-induced apoptosis. Clin. Cancer Res. 2001, 7, 1043–1051. [Google Scholar] [PubMed]
- Feldman, D.; Krishnan, A.V.; Swami, S.; Giovannucci, E.; Feldman, B.J. The role of vitamin D in reducing cancer risk and progression. Nat. Rev. Cancer 2014, 14, 342–357. [Google Scholar] [CrossRef] [PubMed]
- Kovalenko, P.L.; Zhang, Z.; Cui, M.; Clinton, S.K.; Fleet, J.C. 1,25 dihydroxyvitamin D-mediated orchestration of anticancer, transcript-level effects in the immortalized, non-transformed prostate epithelial cell line, RWPE1. BMC Genom. 2010, 11, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCray, T.; Pacheco, J.V.; Loitz, C.C.; Garcia, J.; Baumann, B.; Schlicht, M.J.; Valyi-Nagy, K.; Abern, M.R.; Nonn, L. Vitamin D sufficiency enhances differentiation of patient-derived prostate epithelial organoids. iScience 2021, 24, 101974. [Google Scholar] [CrossRef] [PubMed]
- Giangreco, A.A.; Vaishnav, A.; Wagner, D.; Finelli, A.; Fleshner, N.; Van der Kwast, T.; Vieth, R.; Nonn, L. Tumor suppressor microRNAs, miR-100 and -125b, are regulated by 1,25-dihydroxyvitamin D in primary prostate cells and in patient tissue. Cancer Prev. Res. 2013, 6, 483–494. [Google Scholar] [CrossRef] [Green Version]
- Milani, C.; Katayama, M.L.; de Lyra, E.C.; Welsh, J.; Campos, L.T.; Brentani, M.M.; Maciel Mdo, S.; Roela, R.A.; del Valle, P.R.; Goes, J.C.; et al. Transcriptional effects of 1,25 dihydroxyvitamin D3 physiological and supra-physiological concentrations in breast cancer organotypic culture. BMC Cancer 2013, 13, 119. [Google Scholar] [CrossRef]
- Ajibade, A.A.; Kirk, J.S.; Karasik, E.; Gillard, B.; Moser, M.T.; Johnson, C.S.; Trump, D.L.; Foster, B.A. Early growth inhibition is followed by increased metastatic disease with vitamin D (calcitriol) treatment in the TRAMP model of prostate cancer. PLoS ONE 2014, 9, e89555. [Google Scholar] [CrossRef]
- Anisiewicz, A.; Pawlik, A.; Filip-Psurska, B.; Wietrzyk, J. Differential impact of calcitriol and its analogs on tumor stroma in young and aged ovariectomized mice bearing 4T1 mammary gland cancer. Int. J. Mol. Sci. 2020, 21, 6359. [Google Scholar] [CrossRef]
- Pawlik, A.; Anisiewicz, A.; Filip-Psurska, B.; Nowak, M.; Turlej, E.; Trynda, J.; Banach, J.; Gretkierewicz, P.; Wietrzyk, J. Calcitriol and its analogs establish the immunosuppressive microenvironment that drives metastasis in 4T1 mouse mammary gland cancer. Int. J. Mol. Sci. 2018, 19, 2116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szymczak, I.; Pawliczak, R. The active metabolite of vitamin D3 as a potential immunomodulator. Scand. J. Immunol. 2016, 83, 83–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christakos, S.; Dhawan, P.; Verstuyf, A.; Verlinden, L.; Carmeliet, G. Vitamin D: Metabolism, molecular mechanism of action, and pleiotropic effects. Physiol. Rev. 2016, 96, 365–408. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stephan, C.; Ralla, B.; Bonn, F.; Diesner, M.; Lein, M.; Jung, K. Vitamin D Metabolites in Nonmetastatic High-Risk Prostate Cancer Patients with and without Zoledronic Acid Treatment after Prostatectomy. Cancers 2022, 14, 1560. https://doi.org/10.3390/cancers14061560
Stephan C, Ralla B, Bonn F, Diesner M, Lein M, Jung K. Vitamin D Metabolites in Nonmetastatic High-Risk Prostate Cancer Patients with and without Zoledronic Acid Treatment after Prostatectomy. Cancers. 2022; 14(6):1560. https://doi.org/10.3390/cancers14061560
Chicago/Turabian StyleStephan, Carsten, Bernhard Ralla, Florian Bonn, Max Diesner, Michael Lein, and Klaus Jung. 2022. "Vitamin D Metabolites in Nonmetastatic High-Risk Prostate Cancer Patients with and without Zoledronic Acid Treatment after Prostatectomy" Cancers 14, no. 6: 1560. https://doi.org/10.3390/cancers14061560