Real World Cost-Effectiveness Analysis of Population Screening for BRCA Variants among Ashkenazi Jews Compared with Family History-Based Strategies
Abstract
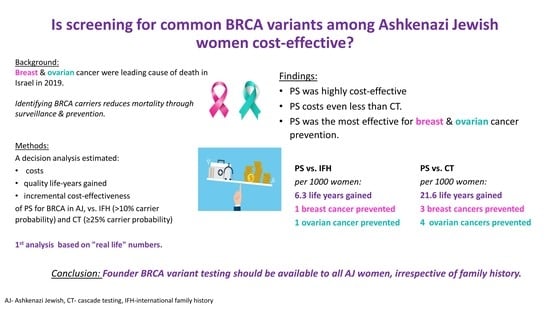
:Simple Summary
Abstract
1. Introduction
- A.
- B.
- Cascade testing (CT), as outlined in Israeli Ministry of Health Guidelines (IMOH) directives [34], requiring probability of at least 25% for identifying a BRCA variant for testing. Women fulfilling these criteria are first/second degree relatives of known carriers. CT is an existing strategy implemented elsewhere, recently described as “an emerging strategy” [35].
2. Materials and Methods
Decision Tree Parameters
- A.
- Identifying carriers:
- B.
- Surveillance and prevention-probabilities and costs:
- C.
- Cancer risks:
- D.
- Overall healthcare costs of women with BC, OC and unaffected:
- E.
- Life expectancy and QALY:
- F.
- Sensitivity analysis
3. Results
3.1. Cost-Utility Analysis
3.2. Sensitivity Analysis
4. Discussion
4.1. Main Findings
4.2. Comparison with Previous Studies
4.3. Sensitivity Analysis
4.4. Strengths
4.5. Limitations
4.6. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Israeli Ministy of Health. Age Adjusted Mortality Rate per 100,000 Persons: Detailed Causes. Available online: https://statistics.health.gov.il/views/DeathCauses/Mortalityratedetailedcauses?%3Aembed=y&Language%20Desc=English (accessed on 1 July 2022).
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- King, M.C.; Marks, J.H.; Mandell, J.B. New York Breast Cancer Study Group. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science 2003, 302, 643–646. [Google Scholar] [CrossRef] [PubMed]
- Hirsh-Yechezkel, G.; Chetrit, A.; Lubin, F.; Friedman, E.; Peretz, T.; Gershoni, R.; Rizel, S.; Struewing, J.P.; Modan, B. Population attributes affecting the prevalence of BRCA variant carriers in epithelial ovarian cancer cases in Israel. Gynecol. Oncol. 2003, 89, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Moslehi, R.; Chu, W.; Karlan, B.; Fishman, D.; Risch, H.; Fields, A.; Smotkin, D.; Ben-David, Y.; Rosenblatt, J.; Russo, D.; et al. BRCA1 and BRCA2 variant analysis of 208 Ashkenazi Jewish women with ovarian cancer. Am. J. Hum. Genet. 2000, 66, 1259–1272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Struewing, J.; Abeliovich, D.; Peretz, T.; Avishai, N.; Kaback, M.M.; Collins, F.S.; Brody, L.C. The carrier frequency of the BRCA1 185delAG variant is approximately 1 percent in Ashkenazi Jewish individuals. Nat. Genet. 1995, 11, 198–200. [Google Scholar] [CrossRef]
- Struewing, J.P.; Hartge, P.; Wacholder, S.; Baker, S.M.; Berlin, M.; McAdams, M.; Timmerman, M.M.; Brody, L.C.; Tucker, M.A. The risk of cancer associated with specific mutations of BRCA1 and BRCA2 among Ashkenazi Jews. N. Engl. J. Med. 1997, 336, 1401–1408. [Google Scholar] [CrossRef]
- Oddoux, C.; Struewing, J.P.; Clayton, C.M.; Pramanik, S.; Oratz, R.; Schnabel, F.; Guha, S.; LeDuc, C.; Campbell, C.L.; Klugman, S.D.; et al. The carrier frequency of the BRCA2 6174delT variant among Ashkenazi Jewish individuals is approximately 1%. Nat. Genet. 1996, 14, 188–190. [Google Scholar] [CrossRef]
- Rosenthal, E.; Moyes, K.; Arnell, C.; Evans, B.; Wenstrup, R.J. Incidence of BRCA1 and BRCA2 non-founder variants in patients of Ashkenazi Jewish ancestry. BC Res. Treat. 2015, 149, 223–227. [Google Scholar] [CrossRef]
- Frank, T.S.; Deffenbaugh, A.M.; Reid, J.E.; Hulick, M.; Ward, B.E.; Lingenfelter, B.; Gumpper, K.L.; Scholl, T.; Tavtigian, S.V.; Pruss, D.R.; et al. Clinical Characteristics of Individuals with germline mutations in BRCA1 and BRCA2: Analysis of 10,000 Individuals. J. Clin. Oncol. 2002, 20, 1480–1490. [Google Scholar] [CrossRef]
- Gabai-Kapara, E.; Lahad, A.; Kaufman, B.; Friedman, E.; Segev, S.; Renbaum, P.; Beeri, R.; Gal, M.; Grinshpun-Cohen, J.; Djemal, K.; et al. Population-based screening for breast and ovarian cancer risk due to BRCA1 and BRCA2. Proc. Natl. Acad. Sci. USA 2014, 111, 14205–14210. [Google Scholar] [CrossRef]
- Lieberman, S.; Tomer, A.; Ben-chetrit, A.; Olsha, O.; Strano, S.; Beeri, R.; Koka, S.; Fridman, H.; Djemal, K.; Glick, I.; et al. Population screening for BRCA1/BRCA2 founder mutations in Ashkenazi Jews: Proactive recruitment compared with self-referral. Genet. Med. 2017, 19, 754–762. [Google Scholar] [CrossRef] [Green Version]
- Møller, P.; Hagen, A.I.; Apold, J.; Maehle, L.; Clark, N.; Fiane, B.; Løvslett, K.; Hovig, E.; Vabø, A. Genetic epidemiology of BRCA mutations—family history detects less than 50% of the variant carriers. Eur. J. Cancer 2007, 43, 1713–1717. [Google Scholar] [CrossRef]
- Weitzel, J.N.; Cullinane, C.A.; Gambol, P.J.; Gambol, P.J.; Culver, J.O.; Blazer, K.R.; Palomares, M.R.; Lowstuter, K.J.; MacDonald, D.J. Limited Family Structure and in Single Cases of breast cancer. JAMA 2007, 297, 2587–2595. [Google Scholar] [CrossRef] [Green Version]
- Metcalfe, K.A.; Poll, A.; Royer, R.; Llacuachaqui, M.; Tulman, A.; Sun, P.; Narod, S.A. Screening for founder mutations in BRCA1 and BRCA2 in unselected jewish women. J. Clin. Oncol. 2010, 28, 387–391. [Google Scholar] [CrossRef]
- Manchanda, R.; Loggenberg, K.; Sanderson, S.; Burnell, M.; Wardle, J.; Gessler, S.; Side, L.; Balogun, N.; Desai, R.; Kumar, A.; et al. Population testing for cancer predisposing BRCA1/BRCA2 mutations in the Ashkenazi-Jewish community: A randomized controlled trial. J. Natl. Cancer Inst. 2014, 107, 379. [Google Scholar] [CrossRef] [Green Version]
- Foulkes, W.D.; Knoppers, B.M.; Turnbull, C. Population genetic testing for cancer susceptibility: Founder mutations to genomes. Nat. Rev. Clin. Oncol. 2015, 13, 41–54. [Google Scholar] [CrossRef]
- Mavaddat, N.; Peock, S.; Frost, D.; Ellis, S.; Platte, R.; Fineberg, E.; Evans, D.G.; Izatt, L.; Eeles, R.A.; Adlard, J.; et al. Cancer risks for BRCA1 and BRCA2 mutation carriers: Results from prospective analysis of EMBRACE. J. Natl. Cancer Inst. 2013, 105, 812–822. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Parmigiani, G. Meta-Analysis of BRCA1 and BRCA2 Penetrance. J. Clin. Oncol. 2007, 25, 1329–1333. [Google Scholar] [CrossRef] [Green Version]
- Daly, M.B.; Pal, T.; Berry, M.P.; Buys, S.S.; Dickson, P.; Domchek, S.M.; Elkhanany, A.; Friedman, S.; Goggins, M.; Hutton, M.L.; et al. Genetic/Familial High-Risk Assessment: Breast, Ovarian, and Pancreatic, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 77–102. [Google Scholar] [CrossRef]
- Marchetti, C.; De Felice, F.; Palaia, I.; Perniola, G.; Musella, A.; Musio, D.; Muzii, L.; Tombolini, V.; Panici, P.B. A meta-analysis on impact on ovarian cancer risk and all cause mortality in BRCA 1 and BRCA 2 variant carriers. BMC Womens Health 2014, 14, 150. [Google Scholar] [CrossRef]
- Domchek, S.M.; Friebel, T.M.; Singer, C.F.; Evans, D.G.; Lynch, H.T.; Isaacs, C.; Garber, J.E.; Neuhausen, S.L.; Matloff, E.; Eeles, R.; et al. Association of risk-reducing surgery in BRCA1 or BRCA2 variant carriers with cancer risk and mortality. JAMA 2010, 304, 967–975. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kauff, N.D.; Domchek, S.M.; Friebel, T.M.; Robson, M.E.; Lee, J.; Garber, J.E.; Isaacs, C.; Evans, D.G.; Lynch, H.; Eeles, R.A.; et al. Risk-reducing salpingo-oophorectomy for the prevention of brca1 and brca2 -associated breast and gynecologic cancer: A multicenter, prospective study. J. Clin. Oncol. 2008, 26, 1331–1337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finch, A.P.M.; Lubinski, J.; Møller, P.; Singer, C.F.; Karlan, B.; Senter, L.; Rosen, B.; Maehle, L.; Ghadirian, P.; Cybulski, C.; et al. Impact of oophorectomy on cancer incidence and mortality in women with a BRCA1 or BRCA2 variant. J. Clin. Oncol. 2014, 32, 1547–1553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, J.M.G.; Jungner, G. Principles and Practice of Screening for Disease; Public Health Papers No 34; World Health Organization: Geneva, Switzerland, 1968. [Google Scholar]
- Murray, M.F.; Evans, J.P.; Angrist, M.; Chan, K.; Uhlmann, W.R.; Doyle, D.L.; Fullerton, S.M.; Ganiats, T.G.; Hagenkord, J.; Imhof, S.; et al. A Proposed Approach for Implementing Genomics-Based Screening Programs for Healthy Adults. NAM Perspectives; 2018 Discussion Paper; National Academy of Medicine: Washington, DC, USA, 2018. [Google Scholar] [CrossRef] [Green Version]
- Hadar, T.; Mor, P.; Amit, G.; Lieberman, S.; Gekhtman, D.; Rabinovitch, R.; Levy-Lahad, E. Presymptomatic awareness of germline pathogenic brca mutations and associated outcomes in women with breast cancer. JAMA Oncol. 2020, 6, 1460–1463. [Google Scholar] [CrossRef] [PubMed]
- Žilovič, D.; Čiurlienė, R.; Sabaliauskaitė, R.; Jarmalaitė, S. Future screening prospects for ovarian cancer. Cancers 2021, 13, 3840. [Google Scholar] [CrossRef]
- Nelson, H.D.; Kari, T.; Arpana, N.; Christina, B.; Humphrey, L.; Chan, B.K. Screening for BC: Systematic evidence review update for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2009, 151, 727–737. [Google Scholar] [CrossRef] [Green Version]
- D’Andrea, E.; Marzuillo, C.; De Vito, C.; Di Marco, M.; Pitini, E.; Vacchio, M.R.; Villari, P. Which BRCA genetic testing programs are ready for implementation in health care? A systematic review of economic evaluations. Genet. Med. 2016, 18, 1171–1180. [Google Scholar] [CrossRef] [Green Version]
- Michaan, N.; Leshno, M.; Safra, T.; Sonnenblick, A.; Laskov, I.; Grisaru, D. Cost effectiveness of whole population BRCA genetic screening for cancer prevention in Israel. Cancer Prev. Res. 2021, 14, 455–462. [Google Scholar] [CrossRef]
- International Breast Cancer Intervention Study (IBIS). BC Risk Evaluation Tool. Available online: https://ibis.ikonopedia.com/ (accessed on 1 May 2022).
- Kenan, E.S.; Friger, M.; Shochat-Bigon, D.; Schayek, H.; Bernstein-Molho, R.; Friedman, E. Accuracy of risk prediction models for BC and BRCA1/BRCA2 variant carrier probabilities in Israel. Anticancer Res. 2018, 38, 4557–4563. [Google Scholar] [CrossRef]
- Bolovitz, Y. Ministry of Health Protocol Review MOH 12/2004. Available online: http://www.health.gov.il/hozer/mr12_2004.pdf (accessed on 1 May 2022).
- Offit, K.; Tkachuk, K.A.; Stadler, Z.K.; Walsh, M.F.; Diaz-Zabala, H.; Levin, J.D.; Steinsnyder, Z.; Ravichandran, V.; Sharaf, R.N.; Frey, M.K.; et al. Cascading after peridiagnostic cancer genetic testing: An alternative to population-based screening. J. Clin. Oncol. 2020, 38, 1398–1408. [Google Scholar] [CrossRef]
- Israel Ministry of Health. 2019 Price List. Available online: https://www.health.gov.il/Subjects/Finance/Taarifon/Pages/PriceList.aspx (accessed on 1 May 2022).
- Tufts CEA Registry. Available online: http://healtheconomics.tuftsmedicalcenter.org/cear2n/search/search.aspx (accessed on 1 May 2022).
- Manchanda, R.; Blyuss, O.; Gaba, F.; Gordeev, V.S.; Jacobs, C.; Burnell, M.; Gan, C.; Taylor, R.; Turnbull, C.; Legood, R.; et al. Current detection rates and time-to-detection of all identifiable BRCA carriers in the Greater London population. J. Med. Gen. 2018, 55, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, S.; Lahad, A.; Tomer, A.; Koka, S.; BenUziyahu, M.; Raz, A.; Levy-Lahad, E. Familial communication and cascade testing among relatives of BRCA population screening participants. Genet. Med. 2018, 20, 1446–1454. [Google Scholar] [CrossRef] [PubMed]
- Shimon, P.S.; Laitman, Y.; Vaisman, Y.; Helpman, L.; Gitly, M.; Paluch Shimon, S.; Berger, R.; Cohen, L.; Narod, S.A.; Friedman, E. Rates of risk-reducing surgery in Israeli BRCA1 and BRCA2 mutationcarriers. Clin. Genet. 2014, 85, 68–71. [Google Scholar]
- Israel Cancer Association. BRCA Carrier Clinics. Available online: http://www.cancer.org.il/template/default.aspx?PageId=8181 (accessed on 1 July 2022).
- Smith, M.J.; Gerber, D.; Olsen, A.; Khouri, O.R.; Wang, Y.; Liu, M.; Smith, J.; Pothuri, B. Uptake and timing of risk-reducing salpingo-oophorectomy among patients with BRCA1 and BRCA2 mutations. Am. J. Obstet. Gynecol. 2021, 225, 508. [Google Scholar] [CrossRef] [PubMed]
- Israel Ministry of Health. Ovarian Cancer Registry. 2016. Available online: http://www.health.gov.il/PublicationsFiles/Ovarian_cancer2013.pdf (accessed on 1 July 2022).
- Israel Ministry of Health. BC Registry. 2018. Available online: https://www.health.gov.il/PublicationsFiles/breast_cancer_SEPT2018.pdf (accessed on 1 July 2022).
- National Institutes of Health. SEER. Available online: https://seer.cancer.gov/statfacts/html/breast.html (accessed on 1 May 2022).
- Klang, S.H.; Hammerman, A.; Liebermann, N.; Efrat, N.; Doberne, J.; Hornberger, J. Economic implications of 21-gene BC risk assay from the perspective of an Israeli-managed healthcare organization. Value Health 2010, 13, 381–387. [Google Scholar] [CrossRef] [Green Version]
- Manchanda, R.; Patel, S.; Antoniou, A.C.; Levy-Lahad, E.; Turnbull, C.; Evans, D.G.; Hopper, J.L.; Macinnis, R.J.; Menon, U.; Jacobs, I.; et al. Cost-effectiveness of population based BRCA testing with varying Ashkenazi Jewish ancestry. Am. J. Obstet. Gynecol. 2017, 217, 578. [Google Scholar] [CrossRef]
- Israeli Central Bureau of Statistics. Available online: https://www.cbs.gov.il/en/Pages/default.aspx (accessed on 1 July 2022).
- Israel Ministry of Health. Relative Survival of Cancer Patients in Israel. 2015. Available online: https://www.health.gov.il/UnitsOffice/ICDC/Chronic_Diseases/Cancer/Pages/Relative_Survival_of_Cancer_Patients.aspx (accessed on 1 July 2022).
- Manchanda, R.; Legood, R.; Burnell, M.; McGuire, A.; Raikou, M.; Loggenberg, K.; Wardle, J.; Sanderson, S.; Gessler, S.; Side, L.; et al. Cost-effectiveness of population screening for BRCA mutations in Ashkenazi Jewish women compared with family history-based testing. J. Natl. Cancer Inst. 2015, 107, 380. [Google Scholar] [CrossRef]
- Rebbeck, T.R.; Kauff, N.D.; Domchek, S.M. Meta-analysis of Risk Reduction Estimates Associated With risk-reducing salpingo- oophorectomy in BRCA1 or BRCA2 variant carriers. JNCI 2009, 101, 80–87. [Google Scholar] [CrossRef]
- Engel, C.; Rhiem, K.; Hahnen, E. German Consortium for Hereditary Breast and Ovarian Cancer (GC-HBOC). Prevalence of pathogenic BRCA1/2 germline mutations among 802 women with unilateral triple-negative breast cancer without family cancer history. BMC Cancer 2018, 18, 265. [Google Scholar] [CrossRef] [Green Version]
- Cheon, J.Y.; Mozersky, J.; Cook-Deegan, R. Variants of uncertain significance in BRCA: A harbinger of ethical and policy issues to come? Genome Med. 2014, 6, 121. [Google Scholar] [CrossRef]
- Eleje, G.U.; Eke, A.C.; Ezebialu, I.U.; Ikechebelu, J.I.; Ugwu, E.O.; Okonkwo, O.O. Risk-reducing bilateral salpingo-oophorectomy in women with BRCA1 or BRCA2 mutations. Cochrane Database Syst. Rev. 2018, 8, 8. [Google Scholar] [CrossRef]
- World Health Organization. Thresholds for the Cost–Effectiveness of Interventions: Alternative Approaches. Available online: https://www.who.int/bulletin/volumes/93/2/14-138206/en/ (accessed on 1 July 2022).
- Heemskerk-Gerritsen, B.A.M.; Seynaeve, C.; van Asperen, C.J.; Ausems, M.G.; Collée, J.M.; van Doorn, H.C.; Gomez Garcia, E.B.; Kets, C.M.; van Leeuwen, F.E.; Meijers-Heijboer, H.E.; et al. Correspondence RE: BC risk after salpingo-oophorectomy in healthy BRCA1 / 2 mutation carriers: Revisiting the evidence for risk reduction. J. Natl. Cancer Inst. 2015, 107, 6–7. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://www.gov.uk/government/publications/breast-screening-higher-risk-women-surveillance-protocols/protocols-for-surveillance-of-women-at-higher-risk-of-developing-breast-cancer (accessed on 1 July 2022).
- Available online: https://www.nice.org.uk/guidance/cg164 (accessed on 1 July 2022).
- Manchanda, R.; Sun, L.; Patel, S.; Evans, O.; Wilschut, J.; De Freitas Lopes, A.C.; Gaba, F.; Brentnall, A.; Duffy, S.; Cui, B.; et al. Economic evaluation of population based BRCA1/BRCA2 mutation testing across multiple countries and health systems. Cancers 2020, 12, 1929. [Google Scholar] [CrossRef]
- Engel, N.J.; Gordon, P.; Thull, D.L.; Dudley, B.; Herstine, J.; Jankowitz, R.C.; Zorn, K.K. A multidisciplinary clinic for individualizing management of patients at increased risk for breast and gynecologic cancer. Fam. Cancer 2012, 11, 419–427. [Google Scholar] [CrossRef]
- Manchanda, R.; Gaba, F. Population based testing for primary prevention: A systematic review. Cancers 2018, 10, 424. [Google Scholar] [CrossRef] [Green Version]
- Terkelsen, T.; Christensen, L.L.; Fenton, D.C.; Jensen, U.B.; Sunde, L.; Thomassen, M.; Skytte, A.B. Population frequencies of pathogenic alleles of BRCA1 and BRCA2: Analysis of 173 Danish breast cancer pedigrees using the BOADICEA model. Fam. Cancer 2019, 18, 381–388. [Google Scholar] [CrossRef]
- Kemp, Z.; Turnbull, A.; Yost, S.; Seal, S.; Mahamdallie, S.; Poyastro-Pearson, E.; Warren-Perry, M.; Eccleston, A.; Tan, M.M.; Teo, S.H.; et al. Evaluation of cancer-based criteria for use in mainstream BRCA1 and BRCA2 genetic testing in patients with breast cancer. JAMA Open 2019, 2, e194428. [Google Scholar] [CrossRef] [Green Version]
- Drohan, B.; Roche, C.A.; Cusack, J.C., Jr.; Hughes, K.S. Hereditary breast and Ovarian cancer and other hereditary syndromes: Using technology to identify carriers. Ann. Surg. Oncol. 2012, 19, 1732–1737. [Google Scholar] [CrossRef]
- Grann, R.; Heitjan, D.; Antman, K.H.; Neugut, A.I. Benefits and costs of screening Ashkenazi Jewish women for BRCA1 and BRCA2. J. Clin. Oncol. 1999, 17, 494–500. [Google Scholar] [CrossRef]
- Rubinstein, W.S.; Jiang, H.; Dellefave, L.; Rademaker, A.W. Cost-effectiveness of population-based BRCA1/2 testing and OC prevention for Ashkenazi Jews: A call for dialogue. Genet. Med. 2009, 11, 629–639. [Google Scholar] [CrossRef]
- Patel, S.; Legood, R.; Evans, D.G.; Turnbull, C.; Antoniou, A.C.; Menon, U.; Jacobs, I.; Manchanda, R. Cost effectiveness of population based BRCA1 founder mutation testing in Sephardi Jewish women. Am. J. Obstet. Gynecol. 2018, 218, 431.e1–431.e12. [Google Scholar] [CrossRef] [PubMed]
- Meshkani, Z.; Aboutorabi, A.; Moradi, N.; Langarizadeh, M.; Motlagh, A.G. Population or family history based BRCA gene tests of breast cancer? A systematic review of economic evaluations. Hered Cancer Clin. Pract. 2021, 19, 35. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, K.; Eisen, A.; Senter, L.; Armel, S.; Bordeleau, L.; Meschino, W.S.; Pal, T.; Lynch, H.T.; Tung, N.M.; Kwong, A.; et al. International trends in the uptake of cancer risk reduction strategies in women with a BRCA1 or BRCA2 mutation. Br. J. Cancer 2019, 121, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Domchek, S.M. Risk-Reducing Mastectomy in BRCA1 and BRCA2 Variant Carriers: A Complex Discussion. JAMA 2019, 321, 27. [Google Scholar] [CrossRef] [PubMed]
- Heemskerk-Gerritsen, B.A.M.; Jager, A.; Koppert, L.B.; Obdeijn, A.I.; Collée, M.; Meijers-Heijboer, H.E.J.; Jenner, D.J.; Oldenburg, H.S.A.; van Engelen, K.; de Vries, J.; et al. Survival after bilateral risk-reducing mastectomy in healthy BRCA1 and BRCA2 mutation carriers. BC Res. Treat. 2019, 177, 723–733. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Bao, Y.; Riaz, M.; Tiller, J.; Liew, D.; Zhuang, X.; Amor, D.J.; Huq, A.; Petelin, L.; Nelson, M.; et al. Population genomic screening of all young adults in a health-care system: A cost-effectiveness analysis. Genet. Med. 2019, 21, 1958–1968. [Google Scholar] [CrossRef] [Green Version]
- Abul-Husn, N.S.; Soper, E.R.; Odgis, J.A.; Cullina, S.; Bobo, D.; Moscati, A.; Rodriguez, J.E.; CBIPM Genomics Team; Regeneron Genetics Center; Loos, R.J.F.; et al. Exome sequencing reveals a high prevalence of BRCA1 and BRCA2 founder mutations in a diverse population-based biobank. Genome Med. 2019, 12, 2. [Google Scholar] [CrossRef]
- Manchanda, R.; Patel, S.; Gordeev, V.S.; Antoniou, A.C.; Smith, S.; Lee, A.; Hopper, J.L.; MacInnis, R.J.; Turnbull, C.; Ramus, S.J.; et al. Cost-effectiveness of population-based BRCA1, BRCA2, RAD51C, RAD51D, BRIP1, PALB2 mutation testing in unselected general population women. J. Natl. Cancer Inst. 2018, 110, 714.e25. [Google Scholar] [CrossRef]
- Lacaze, P.; Tiller, J.; Bao, Y.; Riaz, M.; Winship, I.; Zhang, L. Response to Veenstra. Genet. Med. 2019, 21, 2842.e3. [Google Scholar] [CrossRef]



| Variable | Value | Min | Max | |
|---|---|---|---|---|
| Testing rates | ||||
| P1 | Population screening (PS) [12] | 67% | 50% | 100% |
| P2 | Cascade testing (CT) [39] | 0.68% | 0.2% | 1% |
| P3 | IFH strategy [11] | 11% | 5% | 15% |
| Carrier prevalence | ||||
| P4 | General AJ population [11] | 2.5% | 1.5% | 3% |
| P5 | CT [39] | 41.7% | 25% | 50% |
| P6 | IFH-fulfilling testing criteria [12] | 3.9% | 2.45% | 9% |
| P7 | IFH-not fulfilling testing criteria [12] | 1.7% | 1.2% | 2.04% |
| Risk reducing surgery and cancer risk | ||||
| P8 | RRSO Compliance [41] | 83.5% | 47% | 90% |
| P9 | RRM Compliance | 6% | 3% | 56% |
| P10 | OC risk reduction post RRSO [21] | 96% | 80% | 96% |
| P11 | Carrier-OC risk [11] | 31.3% | 16% | 30% |
| P12 | BC risk reduction post RRSO & RRM [50,51] | 95% | 78% | 99% |
| P13 | Carrier-BC risk [11] | 43% | 31.3% | 53% |
| P14 | BC risk reduction post RRM [52,53] change 61 to ref from ref section listed as 63 (manchannd 2015), change 75 to ref from ref section listed as 22 (domchek 2010) | 90% | 44% | 90% |
| P15 | BC risk reduction post RRSO [52,54] | 50% | 0 | 50% |
| P16 | Non carrier-BC risk [44,45] | 13% | 11% | 14% |
| P17 | Non carrier-OC risk [43,45] | 1.5% | 0.8% | 1.5% |
| Health Service | Cost (NIS) | Cost (USD) 1 |
|---|---|---|
| Genetic counseling and testing | ||
| Genetic counseling | 635 | 178 |
| Genetic testing | 635 | 178 |
| Genetic testing-high throughput (PS 2) | 80 | 22 |
| Carriers: Risk reducing surgery | ||
| RRM | 29,650 | 8305 |
| RRSO | 16,110 | 4513 |
| Carriers: Surveillance modalities | ||
| CA-125 (twice a year) | 96 | 27 |
| Pelvic ultrasound (twice a year) | 650 | 182 |
| Mammogram (annual) | 277 | 78 |
| MRI (annual) | 2060 | 577 |
| Clinical breast examination (annual) | 283 | 79 |
| Carriers: Total surveillance cost per year 3 | ||
| Unaffected, no RRM/Affected with BC, post diagnosis | 3366 | 943 |
| Unaffected, post RRM | 1029 | 288 |
| Affected with OC, post diagnosis | 2716 | 760 |
| Affected with OC, post diagnosis & post RRM | 379 | 106 |
| Carriers: Discounted cumulative surveillance cost (40–75 years) 3 | ||
| Unaffected, no RRM/Affected with BC, post diagnosis | 121,176 | 33,943 |
| Unaffected, post RRM | 37,044 | 10,376 |
| Affected with OC, post diagnosis | 107,526 | 30,119 |
| Affected with OC & post RRM | 23,394 | 6553 |
| Lifetime Incidence Per 1000 Women by Testing Strategy | |||
|---|---|---|---|
| Cancer | CT | IFH | PS |
| Breast cancer | 138 | 136 | 135 |
| Ovarian cancer | 23 | 20 | 19 |
| Strategy | Cost (USD) | Incremental Cost (USD) | Effectiveness (QALY) | Incremental Effectiveness (Per 1000 Women) | ICER/QALY (USD) |
|---|---|---|---|---|---|
| PS | 26,924 | 26.408 | |||
| IFH (vs. PS) | 26,652 | 272 | 26.402 | 0.0063 (6.3 years) | 42,261 |
| CT (vs. IFH) | 26,991 | −339 | 26.386 | 0.016 (16 years) | −21,187.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michaelson-Cohen, R.; Cohen, M.J.; Cohen, C.; Greenberg, D.; Shmueli, A.; Lieberman, S.; Tomer, A.; Levy-Lahad, E.; Lahad, A. Real World Cost-Effectiveness Analysis of Population Screening for BRCA Variants among Ashkenazi Jews Compared with Family History-Based Strategies. Cancers 2022, 14, 6113. https://doi.org/10.3390/cancers14246113
Michaelson-Cohen R, Cohen MJ, Cohen C, Greenberg D, Shmueli A, Lieberman S, Tomer A, Levy-Lahad E, Lahad A. Real World Cost-Effectiveness Analysis of Population Screening for BRCA Variants among Ashkenazi Jews Compared with Family History-Based Strategies. Cancers. 2022; 14(24):6113. https://doi.org/10.3390/cancers14246113
Chicago/Turabian StyleMichaelson-Cohen, Rachel, Matan J. Cohen, Carmit Cohen, Dan Greenberg, Amir Shmueli, Sari Lieberman, Ariela Tomer, Ephrat Levy-Lahad, and Amnon Lahad. 2022. "Real World Cost-Effectiveness Analysis of Population Screening for BRCA Variants among Ashkenazi Jews Compared with Family History-Based Strategies" Cancers 14, no. 24: 6113. https://doi.org/10.3390/cancers14246113