Do Patient-Reported Upper-Body Symptoms Predict Breast Cancer-Related Lymphoedema: Results from a Population-Based, Longitudinal Breast Cancer Cohort Study
Abstract
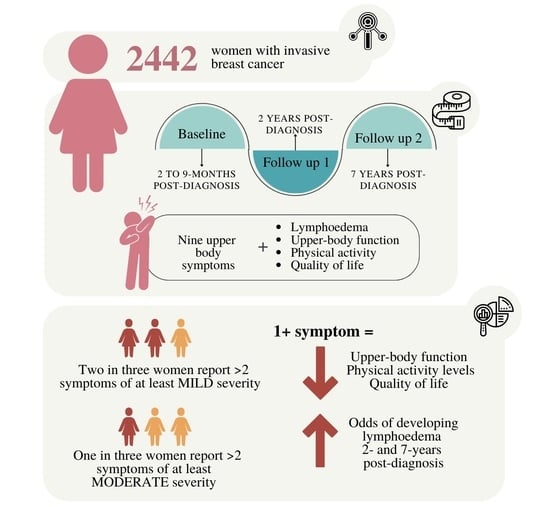
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Outcome Ascertainment
2.1.1. Upper-Body Symptoms
2.1.2. Upper-Body Function
2.1.3. Breast Cancer-Related Lymphoedema
2.1.4. Quality of Life
2.1.5. Physical Activity
2.2. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J.; et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef] [Green Version]
- World Health Organisation. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 7 March 2022).
- Jammallo, L.S.; Miller, C.L.; Horick, N.K.; Skolny, M.N.; O’Toole, J.; Specht, M.C.; Taghian, A.G. Factors associated with fear of lymphedema after treatment for breast cancer. Oncol. Nurs. Forum 2014, 41, 473–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Executive Committee of the International Society of Lymphology. The diagnosis and treatment of peripheral lymphedema: 2020 Consensus Document of the International Society of Lymphology. Lymphology 2020, 53, 3–19. [Google Scholar]
- Dean, L.T.; Moss, S.L.; Ransome, Y.; Frasso-Jaramillo, L.; Zhang, Y.; Visvanathan, K.; Nicholas, L.H.; Schmitz, K.H. “It still affects our economic situation”: Long-term economic burden of breast cancer and lymphedema. Support. Care Cancer 2019, 27, 1697–1708. [Google Scholar] [CrossRef]
- Hayes, S.C.; Rye, S.; Battistutta, D.; Newman, B. Prevalence of upper-body symptoms following breast cancer and its relationship with upper-body function and lymphedema. Lymphology 2010, 43, 178–187. [Google Scholar] [PubMed]
- Taghian, N.R.; Miller, C.L.; Jammallo, L.S.; O’Toole, J.; Skolny, M.N. Lymphedema following breast cancer treatment and impact on quality of life: A review. Crit. Rev. Oncol. Hematol. 2014, 92, 227–234. [Google Scholar] [CrossRef]
- Hayes, S.C.; Rye, S.; Battistutta, D.; DiSipio, T.; Newman, B. Upper-body morbidity following breast cancer treatment is common, may persist longer-term and adversely influences quality of life. Health Qual. Life Outcomes 2010, 8, 92. [Google Scholar] [CrossRef] [Green Version]
- Jørgensen, M.G.; Toyserkani, N.M.; Hansen, F.G.; Bygum, A.; Sørensen, J.A. The impact of lymphedema on health-related quality of life up to 10 years after breast cancer treatment. NPJ Breast Cancer 2021, 7, 70. [Google Scholar] [CrossRef]
- DiSipio, T.D.; Rye, S.M.; Newman, B.P.; Hayes, S.P. Incidence of unilateral arm lymphoedema after breast cancer: A systematic review and meta-analysis. Lancet Oncol. 2013, 14, 500–515. [Google Scholar] [CrossRef]
- Ren, Y.; Kebede, M.A.; Ogunleye, A.A.; Emerson, M.A.; Evenson, K.R.; Carey, L.A.; Hayes, S.C.; Troester, M.A. Burden of lymphedema in long-term breast cancer survivors by race and age. Cancer 2022, 128, 4119–4128. [Google Scholar] [CrossRef] [PubMed]
- Paramanandam, V.S.; Dylke, E.; Clark, G.M.; Daptardar, A.A.; Kulkarni, A.M.; Nair, N.S.; Badwe, R.A.; Kilbreath, S.L. Prophylactic Use of Compression Sleeves Reduces the Incidence of Arm Swelling in Women at High Risk of Breast Cancer–Related Lymphedema: A Randomized Controlled Trial. J. Clin. Oncol. 2022, 40, 2004–2012. [Google Scholar] [CrossRef] [PubMed]
- Hayes, S.C.; Singh, B.; Reul-Hirche, H.; Bloomquist, K.; Johansson, K.; Jönsson, C.; Plinsinga, M.L. The Effect of Exercise for the Prevention and Treatment of Cancer-Related Lymphedema: A Systematic Review with Meta-analysis. Med. Sci. Sports Exerc. 2022, 54, 1389–1399. [Google Scholar] [CrossRef] [PubMed]
- McGee, S.A.; Durham, D.D.; Tse, C.K.; Millikan, R.C. Determinants of breast cancer treatment delay differ for African American and White women. Cancer Epidemiol. Biomark. Prev. 2013, 22, 1227–1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emerson, M.A.; Golightly, Y.M.; Tan, X.; Aiello, A.E.; Reeder-Hayes, K.E.; Olshan, A.F.; Earp, H.S.; Troester, M.A. Integrating access to care and tumor patterns by race and age in the Carolina Breast Cancer Study, 2008-2013. Cancer Causes Control. 2020, 31, 221–230. [Google Scholar] [CrossRef]
- Elm, E.v.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. Strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ 2007, 335, 806–808. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, S.B.; Reeder-Hayes, K.E.; Carey, L.A. Disparities in breast cancer treatment and outcomes: Biological, social, and health system determinants and opportunities for research. Oncologist 2013, 18, 986. [Google Scholar] [CrossRef] [Green Version]
- The Functional Assessment of Chronic Illness Therapy Measurement System (FACIT). Functional Assessment of Cancer Therapy-Breast (FACT-B). Available online: https://www.facit.org/measures/FACT-B (accessed on 2 October 2022).
- Beaton, D.E.; Katz, J.N.; Fossel, A.H.; Wright, J.G.; Tarasuk, V.; Bombardier, C. Measuring the whole or the parts? Validity, reliability, and responsiveness of the Disabilities of the Arm, Shoulder and Hand outcome measure in different regions of the upper extremity. J. Hand. Ther. 2001, 14, 128–146. [Google Scholar] [CrossRef]
- Gabel, C.P.; Yelland, M.; Melloh, M.; Burkett, B. A modified QuickDASH-9 provides a valid outcome instrument for upper limb function. BMC Musculoskelet. Disord. 2009, 10, 161. [Google Scholar] [CrossRef] [Green Version]
- Centers for Disease Control and Prevention. Behavioral Risk Factor Surveillance System Survey Questionnaire; U.S. Department of Health and Human Services, Centers for Disease Control and Prevention: Atlanta, GA, USA, 2001. Available online: http://cdc.gov/brfss/annual_data/annual_2001.htm.8/24/2012 (accessed on 12 October 2022).
- Hair, B.Y.; Tse, C.K.; Olshan, A.F.; Hayes, S.; Bell, M.B. Racial differences in physical activity among breast cancer survivors: Implications for breast cancer care. Cancer 2014, 120, 2174–2182. [Google Scholar] [CrossRef] [Green Version]
- Brucker, P.S.; Yost, K.; Cashy, J.; Webster, K.; Cella, D. General population and cancer patient norms for the Functional Assessment of Cancer Therapy-General (FACT-G). Eval Health Prof. 2005, 28, 192–211. [Google Scholar] [CrossRef] [PubMed]
- Franchignoni, F.; Vercelli, S.; Giordano, A.; Sartorio, F.; Bravini, E.; Ferriero, G. Minimal Clinically Important Difference of the Disabilities of the Arm, Shoulder and Hand Outcome Measure (DASH) and Its Shortened Version (QuickDASH). J. Orthop. Sports Phys. Ther. 2014, 44, 30–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armer, J.M.; Ballman, K.V.; McCall, L.; Ostby, P.L.; Zagar, E.; Kuerer, H.M.; Hunt, K.K.; Boughey, J.C. Factors Associated With Lymphedema in Women With Node-Positive Breast Cancer Treated With Neoadjuvant Chemotherapy and Axillary Dissection. JAMA Surg. 2019, 154, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Ridner, S.H.; Shah, C.; Boyages, J.; Koelmeyer, L.; Ajkay, N.; DeSnyder, S.M.; McLaughlin, S.A.; Dietrich, M.S. L-Dex, arm volume, and symptom trajectories 24 months after breast cancer surgery. Cancer Med. 2020, 9, 5164–5173. [Google Scholar] [CrossRef] [PubMed]
- Blom, K.Y.; Johansson, K.I.; Nilsson-Wikmar, L.B.; Brogårdh, C.B. Early intervention with compression garments prevents progression in mild breast cancer-related arm lymphedema: A randomized controlled trial. Acta Oncol. 2022, 61, 897–905. [Google Scholar] [CrossRef]
- Ridner, S.H.; Dietrich, M.S.; Boyages, J.; Koelmeyer, L.; Elder, E.; Hughes, T.M.; French, J.; Ngui, N.; Hsu, J.; Abramson, V.G.; et al. A Comparison of Bioimpedance Spectroscopy or Tape Measure Triggered Compression Intervention in Chronic Breast Cancer Lymphedema Prevention. Lymphat. Res. Biol. 2022. online ahead of print. [Google Scholar] [CrossRef]
- Stout Gergich, N.L.; Pfalzer, L.A.; McGarvey, C.; Springer, B.; Gerber, L.H.; Soballe, P. Preoperative assessment enables the early diagnosis and successful treatment of lymphedema. Cancer 2008, 112, 2809–2819. [Google Scholar] [CrossRef] [Green Version]
- Rabe, E.; Partsch, H.; Morrison, N.; Meissner, M.H.; Mosti, G.; Lattimer, C.R.; Carpentier, P.H.; Gaillard, S.; Jünger, M.; Urbanek, T.; et al. Risks and contraindications of medical compression treatment-A critical reappraisal. An international consensus statement. Phlebology 2020, 35, 447–460. [Google Scholar] [CrossRef]
- Hayes, S.; Janda, M.; Cornish, B.; Battistutta, D.; Newman, B. Lymphedema secondary to breast cancer: How choice of measure influences diagnosis, prevalence, and identifiable risk factors. Lymphology 2008, 41, 18–28. [Google Scholar]
| Characteristic | N (%) |
|---|---|
| Race | |
| Black | 1289 (52.8%) |
| White | 1153 (47.2%) |
| Age | |
| <50 | 1189 (48.7%) |
| 50+ | 1253 (51.3%) |
| Stage at Diagnosis | |
| I | 1141 (46.8%) |
| II | 1006 (41.2%) |
| III | 293 (12.0%) |
| Treatment Type | |
| Surgery Only | 326 (13.4%) |
| Surgery + Radiation | 617 (25.3%) |
| Surgery + Chemotherapy | 348 (14.3%) |
| Surgery, Radiation, Chemotherapy | 1151 (47.1%) |
| Body mass index | |
| <25 | 676 (27.8%) |
| 25–30 | 1147 (47.2%) |
| 30+ | 609 (25.0%) |
| Physical activity (3 months before diagnosis) | |
| Sedentary | 386 (15.8%) |
| Insufficiently active | 546 (22.4%) |
| Sufficiently active | 1506 (61.8%) |
| Physical activity (median 5 months post-diagnosis) | |
| Sedentary | 1062 (43.5%) |
| Insufficiently active | 561 (23.0%) |
| Sufficiently active | 816 (33.5%) |
| Number of lymph nodes examined median (min, max) | 4 (0, 57) |
| Upper-Body Symptoms | Indicated as at Least Mild in Severity | Indicated as at Least Moderate in Severity | ||
|---|---|---|---|---|
| Baseline a | 2-Years PD | Baseline a | 2-Years PD | |
| N (%) | N (%) | N (%) | N (%) | |
| Pain with movement | 1151 (47.2%) | 959 (44.7%) | 528 (21.7%) | 429 (20.0%) |
| Pain (general) | 1297 (53.3%) | 1101 (51.5%) | 597 (24.5%) | 523 (24.5%) |
| Pain with specific activity | 1327 (54.7%) | 1135 (53.4%) | 652 (26.9%) | 560 (26.3%) |
| Poor range of arm movement | 1028 (42.2%) | 932 (43.6%) | 487 (20.0%) | 462 (21.6%) |
| Numbness | 1045 (43.0%) | 929 (43.3%) | 668 (27.5%) | 539 (25.1%) |
| Stiffness (treated side) | 920 (37.8%) | 828 (38.7%) | 469 (19.3%) | 415 (19.4%) |
| Stiffness (arm, shoulder, hand) | 1122 (46.0%) | 1055 (49.4%) | 523 (21.5%) | 490 (22.9%) |
| Heaviness | 684 (28.1%) | 658 (30.7%) | 358 (14.7%) | 368 (17.2%) |
| Achiness | 990 (40.6%) | 900 (42.0%) | 520 (21.3%) | 476 (22.2%) |
| Tightness | 1052 (43.2%) | 926 (43.2%) | 563 (23.1%) | 479 (22.4%) |
| Tingling | 1155 (47.4%) | 1008 (47.3%) | 613 (25.2%) | 513 (24.1%) |
| Weakness | 1238 (50.8%) | 1116 (52.3%) | 578 (23.7%) | 552 (25.9%) |
| Number of symptoms b: | ||||
| Median (min, max) | 3 (0, 9) | 3 (0, 9) | 1 (0, 9) | 0 (0, 9) |
| 0 | 567 (23.3%) | 482 (22.8%) | 1204 (49.6%) | 1107 (52.3%) |
| 1 | 277 (11.4%) | 264 (12.5%) | 332 (13.7%) | 236 (11.2%) |
| 2 | 215 (8.9%) | 217 (10.3%) | 201 (8.3%) | 191 (9.0%) |
| 3 | 182 (7.5%) | 153 (7.2%) | 128 (5.3%) | 100 (4.7%) |
| 4 | 188 (7.7%) | 118 (5.6%) | 113 (4.7%) | 86 (4.1%) |
| 5 | 164 (6.8%) | 141 (6.7%) | 96 (4.0%) | 68 (3.2%) |
| 6 | 149 (6.1%) | 125 (5.9%) | 79 (3.3%) | 69 (3.3%) |
| 7 | 166 (6.8%) | 131 (6.2%) | 90 (3.7%) | 64 (3.0%) |
| 8 | 197 (8.1%) | 157 (7.4%) | 82 (3.4%) | 54 (2.6%) |
| 9 | 324 (13.3%) | 328 (15.5%) | 104 (4.3%) | 141 (6.7%) |
| Timing of Assessment | Baseline a | 2-Years Post-Diagnosis | 7-Years Post-Diagnosis b | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Upper-body symptoms (at least one symptom of mild severity or higher) | |||||||||
| No | Yes | p Value | No | Yes | p Value | No | Yes | p Value | |
| Upper-body function | |||||||||
| QuickDASH c: median | 2.3 | 22.7 | <0.001 | 2.3 | 18.2 | <0.001 | 2.3 | 29.5 | <0.001 |
| (min, max) | (0.0, 43.2) | (0.0, 97.7) | (0.0, 63.6) | (0.0, 97.7) | (0.0, 55.0) | (2.3, 100.0) | |||
| IQR d | (0.0, 9.1) | (11.4, 38.6) | (0.0, 4.5) | (6.8, 38.6) | (0, 6.8) | (15.9, 50.0) | |||
| Breast cancer-related lymphoedema | |||||||||
| Prevalence N (%) | 6 (1.1) | 157 (8.4) | <0.001 | 22 (4.6) | 414 (25.3) | <0.001 | 98 (12.5) | 314 (35.3) | <0.001 |
| Total physical activity (of moderate intensity or higher) as assessed at baseline a, minutes/week | |||||||||
| median | 90 | 30 | <0.001 | 113 | 40 | <0.001 | 90 | 10 | <0.001 |
| (min, max) | (0, 4200) | (0, 5040) | (0, 4200) | (0, 5040) | (0, 5040) | (0, 3690) | |||
| IQR d | (0, 300) | (0, 210) | (0, 360) | (0, 210) | (0, 280) | (0, 180) | |||
| Sedentary, N (%) | 203 (35.8) | 855 (46.0) | <0.001 | 166 (34.4) | 727 (44.6) | <0.001 | 255 (32.4) | 442 (49.8) | <0.001 |
| Insufficiently active N, (%) | 137 (24.2) | 421 (22.7) | <0.001 | 116 (24.1) | 387 (23.7) | <0.001 | 211 (26.8) | 193 (21.7) | <0.001 |
| Sufficiently Active N, (%) | 227 (40.0) | 583 (31.4) | <0.001 | 200 (41.5) | 518 (31.7) | <0.001 | 321 (40.8) | 254 (28.5) | <0.001 |
| Quality of life and subscales | |||||||||
| Lymphoedema (+4 subscale) | 20.0 (0.3) | 15.0 (4.5) | <0.001 | 19.9 (0.4) | 15.0 (4.8) | <0.001 | |||
| Functional status (FACT TOI) e | 76.4 (13.3) | 62.0 (17.2) | <0.001 | 83.0 (9.3) | 66.9 (17.7) | <0.001 | 81.7 (10.1) | 64.0 (17.5) | <0.001 |
| Overall QoL (FACTG) e | 121.7 (16.9) | 103.5 (22.6) | <0.001 | 129.0 (13.5) | 107.6 (24.2) | <0.001 | 127.0 (14.8) | 103.6 (24.3) | <0.001 |
| Breast cancer QoL (FACTB+4) e | 141.7 (16.9) | 118.5 (25.0) | <0.001 | 149.0 (13.5) | 122.7 (27.2) | <0.001 | |||
| Timing of Assessment | Baseline a | 2-Years Post-Diagnosis | ||||
|---|---|---|---|---|---|---|
| Upper-Body Function b | ||||||
| Better | Poorer | p Value | Better | Poorer | p Value | |
| Symptoms (at least mild in severity) | ||||||
| Median (min, max) IQR c | 1 (0, 9) (0, 3) | 6 (0, 9) (3, 8) | <0.001 | 1 (0, 9) (0, 3) | 7 (0, 9) (0, 3) | <0.001 |
| Symptoms (at least moderate in severity) | ||||||
| median (min, max) IQR c | 0 (0, 7) (0, 0) | 3 (0, 9) (1, 6) | <0.001 | 0 (0, 7) (0, 0) | 3 (0, 9) (1, 7) | <0.001 |
| Presence of breast cancer-related lymphoedema | ||||||
| no | yes | p Value | no | yes | p Value | |
| Symptoms (at least mild in severity) | ||||||
| Median (min, max) IQR c | 3 (0, 9) (1, 7) | 8 (0, 9) (4, 9) | <0.001 | 2 (0, 9) (0, 6) | 8 (0, 9) (4, 9) | <0.001 |
| Symptoms (at least moderate in severity) | ||||||
| Median (min, max) IQR c | 0 (0, 9) (0, 3) | 3 (0, 9) (1, 7) | <0.001 | 0 (0, 9) (0, 2) | 3 (0, 9) (0, 8) | <0.001 |
| Breast Cancer-Related Lymphoedema Prevalence at 2-Years Post-Diagnosis | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of Baseline a Symptoms | Lymphoedema Prevalence N (%) | Unadjusted Model (n = 2165) OR (95% CI) | p Value | Demographic Model b (n = 2150) OR (95% CI) | p value | Clinical Model c (n = 2163) OR (95% CI) | p Value | Full Model d (n = 2148) OR (95% CI) | p Value |
| 0 | 135/1093 (12.4%) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | ||||
| 1–2 | 110/480 (22.9%) | 2.11 (1.60–2.79) | <0.001 | 1.79 (1.33–2.42) | 0.001 | 1.74 (1.30–2.34) | <0.001 | 1.49 (1.09–2.04) | 0.013 |
| 3–4 | 63/211 (29.9%) | 3.02 (2.14–4.27) | <0.001 | 2.26 (1.53–3.33) | <0.001 | 2.36 (1.63–3.40) | <0.001 | 1.75 (1.16–2.63) | 0.008 |
| 5–6 | 39/149 (26.2%) | 2.52 (1.67–3.78) | <0.001 | 1.76 (1.10–2.81) | 0.018 | 1.88 (1.22–2.89) | 0.004 | 1.31 (0.80–2.14) | 0.284 |
| 7–9 | 104/226 (46.0%) | 6.05 (4.40–8.31) | <0.001 | 3.37 (2.14–5.31) | <0.001 | 4.57 (3.26–6.42) | <0.001 | 2.58 (1.60–4.17) | <0.001 |
| Breast cancer-related lymphoedema at 7-years post-diagnosis | |||||||||
| Number of baseline a symptoms | Lymphoedema Prevalence N (%) | Unadjusted Model (n = 1694) OR (95% CI) | Demographic Model b (n = 1684) OR (95% CI) | Clinical Model c (n = 1693) OR (95% CI) | Full model d (n = 1683) OR (95% CI) | ||||
| 0 | 124/877 (14.1 %) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | ||||
| 1–2 | 100/372 (26.9%) | 2.23 (1.66–3.01) | <0.001 | 1.83 (1.32–2.53) | <0.001 | 1.78 (1.30–2.44) | <0.001 | 1.45 (1.03–2.05) | 0.032 |
| 3–4 | 65/165 (39.4%) | 3.95 (2.74–5.69) | <0.001 | 2.86 (1.88–4.35) | <0.001 | 2.97 (2.01–4.39) | <0.001 | 2.07 (1.33–3.23) | 0.001 |
| 5–6 | 32/105 (30.5%) | 2.66 (1.69–4.20) | <0.001 | 1.92 (1.15–3.20) | 0.013 | 1.80 (1.10–2.95) | 0.019 | 1.27 (0.74–2.18) | 0.388 |
| 7–9 | 94/171 (55.0%) | 7.41 (5.19–10.58) | <0.001 | 3.94 (2.38–6.51) | <0.001 | 5.42 (3.69–7.95) | <0.001 | 2.75 (1.61–4.70) | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayes, S.C.; Dunn, M.; Plinsinga, M.L.; Reul-Hirche, H.; Ren, Y.; Laakso, E.-L.; Troester, M.A. Do Patient-Reported Upper-Body Symptoms Predict Breast Cancer-Related Lymphoedema: Results from a Population-Based, Longitudinal Breast Cancer Cohort Study. Cancers 2022, 14, 5998. https://doi.org/10.3390/cancers14235998
Hayes SC, Dunn M, Plinsinga ML, Reul-Hirche H, Ren Y, Laakso E-L, Troester MA. Do Patient-Reported Upper-Body Symptoms Predict Breast Cancer-Related Lymphoedema: Results from a Population-Based, Longitudinal Breast Cancer Cohort Study. Cancers. 2022; 14(23):5998. https://doi.org/10.3390/cancers14235998
Chicago/Turabian StyleHayes, Sandra C., Matthew Dunn, Melanie L. Plinsinga, Hildegard Reul-Hirche, Yumeng Ren, E-Liisa Laakso, and Melissa A. Troester. 2022. "Do Patient-Reported Upper-Body Symptoms Predict Breast Cancer-Related Lymphoedema: Results from a Population-Based, Longitudinal Breast Cancer Cohort Study" Cancers 14, no. 23: 5998. https://doi.org/10.3390/cancers14235998