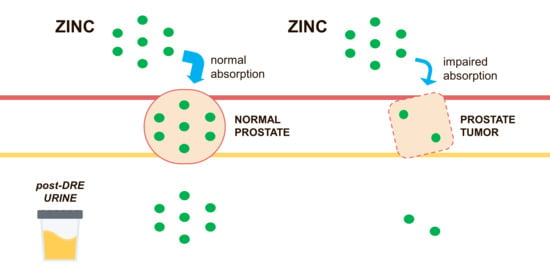
Urinary Zinc Loss Identifies Prostate Cancer Patients
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population and Study Design
2.2. Sample Collection and Processing
2.3. Urine Analysis
2.4. Ethics Statement
2.5. Study Endpoints and Statistical Analyses
3. Results
3.1. Patient Characteristics
3.2. Quantification of Urinary Zinc in Subjects Candidate for Prostate Biopsy
3.3. Evaluation of Urinary ZINC and Routine Parameters
3.4. Diagnostic Accuracy of Urinary Zinc Levels to Identify Clinically Significant Prostate Cancer
3.5. External Validation of the Diagnostic Models
3.6. Urinary zinc and Multiparametric Magnetic Resonance Imaging
3.7. Prostate Cancer Risk Probability Combining Zinc with MRI and Standard Parameters
3.8. Diagnostic Accuracy of Urinary Zinc Levels Evaluation in Patients Undergoing Repeated Prostate Biopsy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salinas, C.A.; Tsodikov, A.; Ishak-Howard, M.; Cooney, K.A. Prostate cancer in young men: An important clinical entity. Nat. Rev. Urol. 2014, 11, 317–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loeb, S.; Bjurlin, M.A.; Nicholson, J.; Tammela, T.L.; Penson, D.F.; Carter, H.B.; Carroll, P.; Etzioni, R. Overdiagnosis and Overtreatment of Prostate Cancer. Eur. Urol. 2014, 65, 1046–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saini, S. PSA and beyond: Alternative prostate cancer biomarkers. Cell. Oncol. 2016, 39, 97–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guazzoni, G.; Lazzeri, M.; Nava, L.; Lughezzani, G.; Larcher, A.; Scattoni, V.; Gadda, G.M.; Bini, V.; Cestari, A.; Buffi, N.M.; et al. Preoperative prostate-specific antigen isoform p2PSA and its derivatives, %p2PSA and prostate health index, predict pathologic outcomes in patients undergoing radical prostatectomy for prostate cancer. Eur. Urol. 2012, 61, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Vickers, A.J.; Vertosick, E.A.; Sjoberg, D.D. Value of a Statistical Model Based on Four Kallikrein Markers in Blood, Commercially Available as 4Kscore, in All Reasonable Prostate Biopsy Subgroups. Eur. Urol. 2018, 74, 535–536. [Google Scholar] [CrossRef]
- Van Neste, L.; Hendriks, R.J.; Dijkstra, S.; Trooskens, G.; Cornel, E.B.; Jannink, S.A.; de Jong, H.; Hessels, D.; Smit, F.P.; Melchers, W.J.; et al. Detection of High-grade Prostate Cancer Using a Urinary Molecular Biomarker-Based Risk Score. Eur. Urol. 2016, 70, 740–748. [Google Scholar] [CrossRef]
- Wei, J.T.; Feng, Z.; Partin, A.W.; Brown, E.; Thompson, I.; Sokoll, L.; Chan, D.W.; Lotan, Y.; Kibel, A.S.; Busby, J.E.; et al. Can urinary PCA3 supplement PSA in the early detection of prostate cancer? J. Clin. Oncol. 2014, 32, 4066–4072. [Google Scholar] [CrossRef] [Green Version]
- Weir, E.G.; Partin, A.W.; Epstein, J.I. Correlation of serum prostate specific antigen and quantitative immunohistochemistry. J. Urol. 2000, 163, 1739–1742. [Google Scholar] [CrossRef]
- Augustin, H.; Hammerer, P.G.; Graefen, M.; Palisaar, J.; Daghofer, F.; Huland, H.; Erbersdobler, A. Characterisation of biomolecular profiles in primary high-grade prostate cancer treated by radical prostatectomy. J. Cancer Res. Clin. Oncol. 2003, 129, 662–668. [Google Scholar] [CrossRef]
- Occhipinti, S.; Mengozzi, G.; Oderda, M.; Zitella, A.; Molinaro, L.; Novelli, F.; Giovarelli, M.; Gontero, P. Low Levels of Urinary PSA Better Identify Prostate Cancer Patients. Cancers 2021, 13, 3570. [Google Scholar] [CrossRef]
- Franklin, R.B.; Milon, B.; Feng, P.; Costello, L.C. Zinc and zinc transporters in normal prostate function and the pathogenesis of prostate cancer. Front. Biosci. 2005, 10, 2230–2239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franz, M.-C.; Anderle, P.; Bürzle, M.; Suzuki, Y.; Freeman, M.; Hediger, M.; Kovacs, G. Zinc transporters in prostate cancer. Mol. Asp. Med. 2013, 34, 735–741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiremath, M.B.; Toragall, M.M.; Satapathy, S.K.; Kadadevaru, G.G. Evaluation of seminal fructose and citric acid levels in men with fertility problem. J. Hum. Reprod. Sci. 2019, 12, 199–203. [Google Scholar] [CrossRef]
- Li, D.; Stovall, D.B.; Wang, W.; Sui, G. Advances of Zinc Signaling Studies in Prostate Cancer. Int. J. Mol. Sci. 2020, 21, 667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortesi, M.; Chechik, R.; Breskin, A.; Vartsky, D.; Ramón, J.; Raviv, G.; Volkov, A.; Fridman, E. Evaluating the cancer detection and grading potential of prostatic-zinc imaging: A simulation study. Phys. Med. Biol. 2009, 54, 781–796. [Google Scholar] [CrossRef]
- Cortesi, M.; Fridman, E.; Volkov, A.; Shilstein SSh Chechik, R.; Breskin, A.; Vartsky, D.; Kleinman, N.; Kogan, G.; Moriel, E.; Gladysh, V.; et al. Clinical assessment of the cancer diagnostic value of prostatic Zinc: A comprehensive nee-dle-biopsy study. Prostate 2008, 68, 994–1006. [Google Scholar] [CrossRef] [PubMed]
- Egevad, L.; Delahunt, B.; Srigley, J.R.; Samaratunga, H. International Society of Urological Pathology (ISUP) grading of prostate cancer—An ISUP consensus on contemporary grading. APMIS 2016, 124, 433–435. [Google Scholar] [CrossRef]
- EAU Guidelines; Edn; presented at the EAU Annual Congress Amsterdam 2022; European Association of Urology: Arnhem, The Netherlands, 2022; ISBN 978-94-92671-16-5.
- Linn, S.; Grunau, P.D. New patient-oriented summary measure of net total gain in certainty for dichotomous diagnostic tests. Epidemiol. Perspect. Innov. 2006, 3, 11. [Google Scholar] [CrossRef] [Green Version]
- Becerra, M.F.; Atluri, V.S.; Bhattu, A.S.; Punnen, S. Serum and urine biomarkers for detecting clinically significant prostate cancer. Urol. Oncol. Semin. Orig. Investig. 2021, 39, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://uroweb.org/guidelines/prostate-cancer (accessed on 1 March 2022).
- Scott, R.; Misser, S.K.; Cioni, D.; Neri, E. PI-RADS v2.1: What has changed and how to report. South Afr. J. Radiol. 2021, 25, 13. [Google Scholar] [CrossRef]
- Fulgham, P.F.; Rukstalis, D.B.; Turkbey, I.B.; Rubenstein, J.N.; Taneja, S.; Carroll, P.R.; Pinto, P.A.; Bjurlin, M.A.; Eggener, S. AUA Policy Statement on the Use of Multiparametric Magnetic Resonance Imaging in the Diagnosis, Staging and Management of Prostate Cancer. J. Urol. 2017, 198, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Malm, J.; Helman, J.; Hogg, P.; Lilja, H. Enzymatic Action of prostate specific antigen PSA or Hk3: Substrate specificity and regulation by Zn, a tight binding inhibitor. Prostate 2000, 45, 132–139. [Google Scholar] [CrossRef]
- Gómez, Y.; Arocha, F.; Espinoza, F.; Fernández, D.; Vásquez, A.; Granadillo, V. Zinc levels in prostatic fluid of patients with prostate pathologies. Investig. Clínica 2007, 48, 287–294. [Google Scholar]
- Zaichick, V.Y.; Sviridova, T.V.; Zaichick, S.V. Zinc in human prostate gland: Normal, hyperplastic and cancerous. Int. Urol. Nepfhrol. 1997, 29, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.C.; Raman, S.S.; Mirak, S.A.; Kwan, L.; Bajgiran, A.M.; Hsu, W.; Maehara, C.K.; Ahuja, P.; Faiena, I.; Pooli, A.; et al. Detection of Individual Prostate Cancer Foci via Multiparametric Magnetic Resonance Imaging. Eur. Urol. 2019, 75, 712–720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norris, J.M.; Simmons, L.A.M.; Kanthabalan, A.; Freeman, A.; McCartan, N.; Moore, C.M.; Punwani, S.; Whitaker, H.C.; Emberton, M.; Ahmed, H.U. Which Prostate Cancers are Undetected by Multiparametric Magnetic Resonance Imaging in Men with Previous Prostate Biopsy? An Analysis from the PICTURE Study. Eur. Urol. Open Sci. 2021, 30, 16–24. [Google Scholar] [CrossRef]
- Sathianathen, N.J.; Omer, A.; Harriss, E.; Davies, L.; Kasivisvanathan, V.; Punwani, S.; Moore, C.M.; Kastner, C.; Barrett, T.; Bergh, R.C.V.D.; et al. Negative Predictive Value of Multiparametric Magnetic Resonance Imaging in the Detection of Clin-ically Significant Prostate Cancer in the Prostate Imaging Reporting and Data System Era: A Systematic Review and Me-ta-analysis. Eur. Urol. 2020, 78, 402–414. [Google Scholar] [CrossRef]
- Mazzone, E.; Gandaglia, G.; Ploussard, G.; Marra, G.; Valerio, M.; Campi, R.; Mari, A.; Minervini, A.; Serni, S.; Moschini, M.; et al. Positive Predictive Value of Prostate Imaging Reporting and Data System Version 2 for the Detection of Clinically Significant Prostate Cancer: A Systematic Review and Meta-analysis. Eur. Urol. 2021, 7, 697–713. [Google Scholar] [CrossRef] [PubMed]
- Borkowetz, A.; Platzek, I.; Toma, M.; Laniado, M.; Baretton, G.; Froehner, M.; Koch, R.; Wirth, M.; Zastrow, S. Comparison of systematic transrectal biopsy to transperineal magnetic resonance imaging/ultrasound-fusion biopsy for the diagnosis of prostate cancer. Br. J. Urol. 2015, 116, 873–879. [Google Scholar] [CrossRef] [Green Version]
- Clavijo Jordan, M.V.; Lo, S.T.; Chen, S.; Preihs, C.; Chirayil, S.; Zhang, S.; Kapur, P.; Li, W.H.; De Leon-Rodriguez, L.M.; Lubag, A.J.; et al. Zinc-sensitive MRI contrast agent detects differential release of Zn(II) ions from the healthy vs. malignant mouse prostate. Proc. Natl. Acad. Sci. USA 2016, 113, E5464–E5471. [Google Scholar] [CrossRef] [Green Version]
- Costello, L.C.; Franklin, R.B. Prostatic fluid electrolyte composition for the screening of prostate cancer: A potential solution to a major problem. Prostate Cancer Prostatic Dis. 2009, 12, 17–24. [Google Scholar] [CrossRef] [PubMed]





| Characteristics | Cohort 1 | Cohort 2 | p |
|---|---|---|---|
| Patients, n | 411 | 212 | - |
| Evaluable samples, n (%) 1 | 394 (96) | 201 (95) | - |
| Age, yr, mean (median; IQR) | 68 (69; 63–74) | 68 (69; 63–73) | ns 2 |
| PSA, ng/mL mean (median; IQR) | 7.2 (6.1; 4.8–8.6) | 7.8 (6.6; 4.7–9.6) | ns |
| DRE abnormal, n (%) | 164 (41.6) | 64 (31.8) | 0.02 |
| PCa diagnosis, n (%) | 207 (52.5) | 127 (63.2) | 0.01 |
| Low risk, n (%) | 17 (8.2) | 12 (9.4) | - |
| Intermediate favorable risk, n (%) | 68 (32.9) | 38 (29.9) | - |
| Intermediate unfavorable risk, n (%) | 67 (32.4) | 38 (29.9) | - |
| High risk, n (%) | 55 (26.6) | 39 (30.7) | - |
| Clinically significant PCa, n (%) | 190 (48.2) | 115 (57.2) | 0.04 |
| Diagnosis | Mean (µg/)mL | Median (p25-p75) | p | |
|---|---|---|---|---|
| Healthy subjects | 1.02 | 0.71 (0.45–1.24) | ref 1 | - |
| Low Risk | 1.93 | 1.48 (0.79–2.31) | ns 2 | ref |
| Int-fav 3 Risk | 0.66 | 0.50 (0.32–0.84) | 0.0163 | 0.0004 |
| Int-unfav 4 Risk | 0.65 | 0.52 (0.27–0.83) | 0.0139 | 0.0004 |
| High Risk | 0.60 | 0.51 (0.32–0.76) | 0.0080 | 0.0002 |
| p for trend | <0.0001 | |||
| Model | OR 1 | 95% CI 2 |
|---|---|---|
| PSA | 1.35 | 0.836–2.172 |
| SOC | 2.39 | 1.582–3.608 |
| Zinc | 2.20 | 1.465–3.300 |
| Zinc + SOC | 3.21 | 2.125–4.845 |
| Model | AUC 1 | SE 2 | 95% CI 3 | p | Spec 4 | Spec 5 | PPV 6 | NPV 7 | NNP 8 | |
|---|---|---|---|---|---|---|---|---|---|---|
| PSA | 0.551 | 0.0290 | 0.501–0.601 | ref | - | 7.5 | 11.3 | 49.1 | 64 | 7.6 |
| SOC | 0.607 | 0.0287 | 0.557–0.656 | ns | ref | 7.5 | 11.3 | 49.2 | 66.7 | 6.3 |
| Zinc | 0.652 | 0.0274 | 0.602–0.699 | 0.0143 | ns | 19 | 32.8 | 52.6 | 82 | 2.9 |
| Zinc + SOC | 0.687 | 0.0265 | 0.639–0.733 | 0.0002 | 0.0011 | 23.4 | 31.8 | 51.3 | 78 | 3.4 |
| Group | AUC 1 | SE 2 | Spec 3 |
|---|---|---|---|
| PSA ≤ ≤4 | 0.589 | 0.0778 | 21.21 |
| 4 < PSA ≤ 10 | 0.642 | 0.034 | 16.79 |
| PSA > 10 | 0.753 | 0.0569 | 26.32 |
| Age ≤ 60 | 0.629 | 0.0705 | 11.21 |
| 60 < Age ≤ 75 | 0.637 | 0.0346 | 21.58 |
| PSA > 75 | 0.697 | 0.0615 | 26.32 |
| Model | AUC | SE | 95% CI | p | Spec | |
|---|---|---|---|---|---|---|
| PSA | 0.558 | 0.0406 | 0.487–0.628 | ref | - | 6.1 |
| SOC | 0.669 | 0.0377 | 0.599–0.734 | 0.0085 | ref | 9.5 |
| Zinc | 0.683 | 0.0387 | 0.614–0.747 | 0.0195 | ns | 37.2 |
| Zinc + SOC | 0.735 | 0.0357 | 0.668–0.795 | 0.0001 | 0.0177 | 41 |
| Model | AUC | SE | 95% CI | p | ||
|---|---|---|---|---|---|---|
| SOC | 0.655 | 0.0366 | 0.599–0.727 | ref | - | - |
| MRI | 0.609 | 0.0314 | 0.542–0.674 | ns | ref | - |
| SOC + MRI | 0.684 | 0.0357 | 0.618–0.744 | ns | 0.0135 | ref |
| Zinc | 0.685 | 0.0366 | 0.620–0.746 | ns | ns | ns |
| Zinc + SOC | 0.761 | 0.0330 | 0.699–0.815 | 0.0014 | 0.0004 | 0.0197 |
| Zinc + SOC + MRI | 0.773 | 0.0320 | 0.712–0.826 | 0.0007 | 0.0001 | 0.0017 |
| PiRADS | PSA | SOC | Zinc | Zinc + SOC |
|---|---|---|---|---|
| 3 | 0.455 | 0.779 * | 0.680 | 0.827 ** |
| 4 | 0.456 | 0.568 | 0.723 **** | 0.730 **** |
| 5 | 0.652 | 0.798 | 0.563 | 0.835 |
| Cut-Off (Probability) | All N (%) | Non-Cancer N (%) | csPCa N (%) | Missed High Risk N (%) | Saved Unnecessary Biopsies N (%) |
|---|---|---|---|---|---|
| 0 | 226 (100) | 93 (100) | 133 (100) | 0 (0) | 0 (0) |
| 25 | 201 (89) | 71 (76) | 130 (98) | 0 (0) | 22 (24) |
| 30 | 196 (87) | 67 (72) | 129 (97) | 0 (0) | 26 (28) |
| 40 | 178 (79) | 56 (60) | 122 (92) | 2 (6) | 37 (40) |
| 45 | 167 (74) | 51 (55) | 116 (87) | 3 (9) | 42 (45) |
| 50 | 152 (67) | 42 (45) | 110 (83) | 5 (14) | 51 (55) |
| Model | AUC | SE | 95% CI | p | |
|---|---|---|---|---|---|
| PSA | 0.538 | 0.065 | 0.441–0.664 | ref | - |
| SOC | 0.608 | 0.062 | 0.486–0.730 | ns | ref |
| Zinc | 0.694 | 0.058 | 0.581–0.808 | ns | ns |
| Zinc + SOC | 0.764 | 0.052 | 0.662–0.685 | 0.002 | 0.009 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maddalone, M.G.; Oderda, M.; Mengozzi, G.; Gesmundo, I.; Novelli, F.; Giovarelli, M.; Gontero, P.; Occhipinti, S. Urinary Zinc Loss Identifies Prostate Cancer Patients. Cancers 2022, 14, 5316. https://doi.org/10.3390/cancers14215316
Maddalone MG, Oderda M, Mengozzi G, Gesmundo I, Novelli F, Giovarelli M, Gontero P, Occhipinti S. Urinary Zinc Loss Identifies Prostate Cancer Patients. Cancers. 2022; 14(21):5316. https://doi.org/10.3390/cancers14215316
Chicago/Turabian StyleMaddalone, Maria Grazia, Marco Oderda, Giulio Mengozzi, Iacopo Gesmundo, Francesco Novelli, Mirella Giovarelli, Paolo Gontero, and Sergio Occhipinti. 2022. "Urinary Zinc Loss Identifies Prostate Cancer Patients" Cancers 14, no. 21: 5316. https://doi.org/10.3390/cancers14215316