Nanoparticles Design for Theranostic Approach in Cancer Disease
Abstract
:Simple Summary
Abstract
1. Introduction
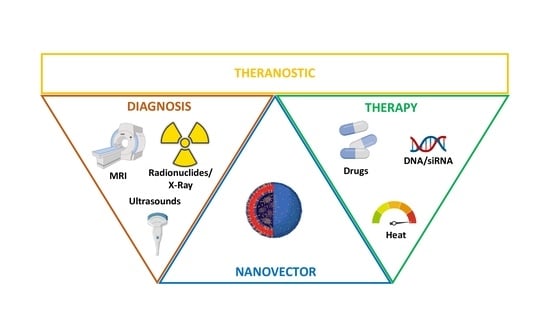
2. NPs for Theranostic Applications
2.1. Inorganic Nanoparticles
2.1.1. Metal Oxide NPs
2.1.2. Metal Organic Frameworks
2.1.3. Gold NPs
2.1.4. Lanthanide-Doped NPs
2.1.5. Silicon Based NPs
2.2. Organic Nanoparticles
2.2.1. Polymeric Nanoparticles
2.2.2. Biological Nanoparticles
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.J.; Pillai, G.G.; Andrade, C.J.; Ligibel, J.A.; Basu, P.; Cohen, L.; Khan, I.A.; Mustian, K.M.; Puthiyedath, R.; Dhiman, K.S.; et al. Integrative oncology: Addressing the global challenges of cancer prevention and treatment. CA Cancer J. Clin. 2022, 72, 144–164. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakara, A.B.; Sundaram, C.; Harikumar, K.B.; Tharakan, S.T.; Lai, O.S.; Sung, B.; Aggarwal, B.B. Cancer is a Preventable Disease That Requires Major Lifestyle Changes. Pharm. Res. 2008, 25, 2097–2116. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Bray, F.; Ferlay, J. Global Cancer Statistics: 2011. CA Cancer J Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Mariotto, A.B.; Etzioni, R.; Hurlbert, M.; Penberthy, L.; Mayer, M. Estimation of the Number of Women Living with Metastatic Breast Cancer in the United States. Cancer Epidemiol. Biomark. Prev. 2017, 26, 809–815. [Google Scholar] [CrossRef]
- Schiffman, J.D.; Fisher, P.G.; Gibbs, P. Early Detection of Cancer: Past, Present, and Future. Am. Soc. Clin. Oncol. Educ. Book 2015, 30, 57–65. [Google Scholar] [CrossRef]
- Hiom, S.C. Diagnosing cancer earlier: Reviewing the evidence for improving cancer survival. Br. J. Cancer 2015, 112, S1–S5. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Ju, D.-T.; Chang, C.-F.; Muralidhar Reddy, P.; Velmurugan, B.K. A review on the effects of current chemotherapy drugs and natural agents in treating non–small cell lung cancer. BioMedicine 2017, 7, 23. [Google Scholar] [CrossRef]
- Belkacemi, Y.; Hanna, N.E.; Besnard, C.; Majdoul, S.; Gligorov, J. Local and Regional Breast Cancer Recurrences: Salvage Therapy Options in the New Era of Molecular Subtypes. Front. Oncol. 2018, 8, 112. [Google Scholar] [CrossRef]
- Hennrich, U.; Benešová, M. [68Ga]Ga-DOTA-TOC: The First FDA-Approved 68Ga-Radiopharmaceutical for PET Imaging. Pharmaceuticals 2020, 13, 38. [Google Scholar] [CrossRef] [Green Version]
- University of Iowa. What Is Theranostics? Available online: https://uihc.org/health-topics/what-theranostics (accessed on 3 September 2022).
- Kievit, F.M.; Zhang, M. Cancer Nanotheranostics: Improving Imaging and Therapy by Targeted Delivery Across Biological Barriers. Adv. Mater. 2011, 23, H217–H247. [Google Scholar] [CrossRef] [PubMed]
- Pandit, C.; Roy, A.; Ghotekar, S.; Khusro, A.; Islam, M.N.; Bin Emran, T.; Lam, S.E.; Khandaker, M.U.; Bradley, D.A. Biological agents for synthesis of nanoparticles and their applications. J. King Saud Univ.-Sci. 2022, 34, 101869. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic: An update. Bioeng. Transl. Med. 2019, 4, e10143. [Google Scholar] [CrossRef] [PubMed]
- Janjua, T.I.; Cao, Y.; Yu, C.; Popat, A. Clinical translation of silica nanoparticles. Nat. Rev. Mater. 2021, 6, 1072–1074. [Google Scholar] [CrossRef] [PubMed]
- Anselmo, A.C.; Mitragotri, S. Nanoparticles in the clinic. Bioeng. Transl. Med. 2016, 1, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Huang, L. Nanoparticles containing insoluble drug for cancer therapy. Biotechnol. Adv. 2013, 32, 778–788. [Google Scholar] [CrossRef]
- Kingston, B.R.; Syed, A.M.; Ngai, J.; Sindhwani, S.; Chan, W.C.W. Assessing micrometastases as a target for nanoparticles using 3D microscopy and machine learning. Proc. Natl. Acad. Sci. USA 2019, 116, 14937–14946. [Google Scholar] [CrossRef]
- Yao, Y.; Zhou, Y.; Liu, L.; Xu, Y.; Chen, Q.; Wang, Y.; Wu, S.; Deng, Y.; Zhang, J.; Shao, A. Nanoparticle-Based Drug Delivery in Cancer Therapy and Its Role in Overcoming Drug Resistance. Front. Mol. Biosci. 2020, 7, 193. [Google Scholar] [CrossRef]
- Sanità, G.; Armanetti, P.; Silvestri, B.; Carrese, B.; Calì, G.; Pota, G.; Pezzella, A.; D’Ischia, M.; Luciani, G.; Menichetti, L.; et al. Albumin-Modified Melanin-Silica Hybrid Nanoparticles Target Breast Cancer Cells via a SPARC-Dependent Mechanism. Front. Bioeng. Biotechnol. 2020, 8, 765. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, F.; Mishra, R.K.; Khan, R. Precision Cancer Nanotherapy: Evolving Role of Multifunctional Nanoparticles for Cancer Active Targeting. J. Med. Chem. 2019, 62, 10475–10496. [Google Scholar] [CrossRef]
- Bazak, R.; Houri, M.; El Achy, S.; Kamel, S.; Refaat, T. Cancer active targeting by nanoparticles: A comprehensive review of literature. J. Cancer Res. Clin. Oncol. 2015, 141, 769–784. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.-H.; Choi, E.; Ellis, E.; Lee, T.-C. Recent advances in gold nanoparticles for biomedical applications: From hybrid structures to multi-functionality. J. Mater. Chem. B 2019, 7, 3480–3496. [Google Scholar] [CrossRef]
- Anani, T.; Rahmati, S.; Sultana, N.; David, A.E. MRI-traceable theranostic nanoparticles for targeted cancer treatment. Theranostics 2021, 11, 579–601. [Google Scholar] [CrossRef]
- Li, S.; Lui, K.-H.; Li, X.; Fang, X.; Lo, W.-S.; Gu, Y.-J.; Wong, W.-T. pH-Triggered Poly(ethylene glycol)–Poly(lactic acid/glycolic acid)/Croconaine Nanoparticles-Assisted Multiplexed Photoacoustic Imaging and Enhanced Photothermal Cancer Therapy. ACS Appl. Bio Mater. 2021, 4, 4152–4164. [Google Scholar] [CrossRef]
- Sonali, M.M.; Viswanadh, M.K.; Singh, R.P.; Agrawal, P.; Mehata, A.K.; Pawde, D.M.; Narendra; Sonkar, R.; Muthu, M.S. Nanotheranostics: Emerging Strategies for Early Diagnosis and Therapy of Brain Cancer. Nanotheranostics 2018, 2, 70–86. [Google Scholar] [CrossRef]
- Zhou, J.; Rao, L.; Yu, G.; Cook, T.R.; Chen, X.; Huang, F. Supramolecular cancer nanotheranostics. Chem. Soc. Rev. 2021, 50, 2839–2891. [Google Scholar] [CrossRef]
- Nam, J.; Won, N.; Bang, J.; Jin, H.; Park, J.; Jung, S.; Jung, S.; Park, Y.; Kim, S. Surface engineering of inorganic nanoparticles for imaging and therapy. Adv. Drug Deliv. Rev. 2013, 65, 622–648. [Google Scholar] [CrossRef]
- Sharma, H.; Kumar, K.; Choudhary, C.; Mishra, P.K.; Vaidya, B. Development and characterization of metal oxide nanoparticles for the delivery of anticancer drug. Artif. Cells Nanomed. Biotechnol. 2016, 44, 672–679. [Google Scholar] [CrossRef]
- Singh, K.R.; Nayak, V.; Singh, J.; Singh, A.K.; Singh, R.P. Potentialities of bioinspired metal and metal oxide nanoparticles in biomedical sciences. RSC Adv. 2021, 11, 24722–24746. [Google Scholar] [CrossRef]
- Samuel, M.S.; Jose, S.; Selvarajan, E.; Mathimani, T.; Pugazhendhi, A. Biosynthesized silver nanoparticles using Bacillus amyloliquefaciens; Application for cytotoxicity effect on A549 cell line and photocatalytic degradation of p-nitrophenol. J. Photochem. Photobiol. B Biol. 2019, 202, 111642. [Google Scholar] [CrossRef] [PubMed]
- Falcaro, P.; Ricco, R.; Yazdi, A.; Imaz, I.; Furukawa, S.; Maspoch, D.; Ameloot, R.; Evans, J.D.; Doonan, C.J. Application of metal and metal oxide nanoparticles@MOFs. Coord. Chem. Rev. 2016, 307, 237–254. [Google Scholar] [CrossRef]
- Vinardell, M.P.; Mitjans, M. Antitumor Activities of Metal Oxide Nanoparticles. Nanomaterials 2015, 5, 1004–1021. [Google Scholar] [CrossRef]
- Kim, M.; Lee, J.-H.; Nam, J.M. Plasmonic Photothermal Nanoparticles for Biomedical Applications. Adv. Sci. 2019, 6, 1900471. [Google Scholar] [CrossRef] [PubMed]
- Sanità, G.; Carrese, B.; Lamberti, A. Nanoparticle Surface Functionalization: How to Improve Biocompatibility and Cellular Internalization. Front. Mol. Biosci. 2020, 7, 587012. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Qiao, R.; Tang, N.; Lu, Z.; Wang, H.; Zhang, Z.; Xue, X.; Huang, Z.; Zhang, S.; Zhang, G.; et al. Active targeting theranostic iron oxide nanoparticles for MRI and magnetic resonance-guided focused ultrasound ablation of lung cancer. Biomaterials 2017, 127, 25–35. [Google Scholar] [CrossRef]
- Quan, Q.; Xie, J.; Gao, H.; Yang, M.; Zhang, F.; Liu, G.; Lin, X.; Wang, A.; Eden, H.S.; Lee, S.; et al. HSA Coated Iron Oxide Nanoparticles as Drug Delivery Vehicles for Cancer Therapy. Mol. Pharm. 2011, 8, 1669–1676. [Google Scholar] [CrossRef]
- Barenholz, Y. Doxil®—The first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, L.; Zhong, H.; Niu, S.; Ding, C.; Lv, S. Iridium oxide nanoparticles-based theranostic probe for in vivo tumor imaging and synergistic chem/photothermal treatments of cancer cells. Chem. Eng. J. 2022, 430, 132675. [Google Scholar] [CrossRef]
- Carrillo-Carrión, C. Nanoscale metal–organic frameworks as key players in the context of drug delivery: Evolution toward theranostic platforms. Anal. Bioanal. Chem. 2020, 412, 37–54. [Google Scholar] [CrossRef]
- Yang, J.; Yang, Y. Metal-organic framework-based cancer theranostic nanoplatforms. VIEW 2020, 1, e20. [Google Scholar] [CrossRef]
- Ding, M.; Liu, W.; Gref, R. Nanoscale MOFs: From synthesis to drug delivery and theranostics applications. Adv. Drug Deliv. Rev. 2022, 12, 114496. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.-X.; Zou, Q.; Sun, S.-K.; Yu, C.; Zhang, X.; Li, R.-J.; Fu, Y.-Y. Theranostic metal–organic framework core–shell composites for magnetic resonance imaging and drug delivery. Chem. Sci. 2016, 7, 5294–5301. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Kumar, A.; Bhamidipati, K.; Puvvada, N.; Sahu, S.K. Facile Strategy to Synthesize Magnetic Upconversion Nanoscale Metal–Organic Framework Composites for Theranostics Application. ACS Appl. Bio Mater. 2020, 3, 869–880. [Google Scholar] [CrossRef]
- Mostafavi, E.; Zarepour, A.; Barabadi, H.; Zarrabi, A.; Truong, L.B.; Medina-Cruz, D. Antineoplastic activity of biogenic silver and gold nanoparticles to combat leukemia: Beginning a new era in cancer theragnostic. Biotechnol. Rep. 2022, 34, e00714. [Google Scholar] [CrossRef]
- Toczek, J.; Sadłocha, M.; Major, K.; Stojko, R. Benefit of Silver and Gold Nanoparticles in Wound Healing Process after Endometrial Cancer Protocol. Biomedicines 2022, 10, 679. [Google Scholar] [CrossRef]
- Nosrati, H.; Seidi, F.; Hosseinmirzaei, A.; Mousazadeh, N.; Mohammadi, A.; Ghaffarlou, M.; Danafar, H.; Conde, J.; Sharafi, A. Prodrug Polymeric Nanoconjugates Encapsulating Gold Nanoparticles for Enhanced X-ray Radiation Therapy in Breast Cancer. Adv. Health Mater. 2022, 11, 2102321. [Google Scholar] [CrossRef]
- Mangadlao, J.D.; Wang, X.; McCleese, C.; Escamilla, M.; Ramamurthy, G.; Wang, Z.; Govande, M.; Basilion, J.P.; Burda, C. Prostate-Specific Membrane Antigen Targeted Gold Nanoparticles for Theranostics of Prostate Cancer. ACS Nano 2018, 12, 3714–3725. [Google Scholar] [CrossRef]
- Yoon, Y.I.; Pang, X.; Jung, S.; Zhang, G.; Kong, M.; Liu, G.; Chen, X. Smart gold nanoparticle-stabilized ultrasound microbubbles as cancer theranostics. J. Mater. Chem. B 2018, 6, 3235–3239. [Google Scholar] [CrossRef] [PubMed]
- Jethva, P.; Momin, M.; Khan, T.; Omri, A. Lanthanide-Doped Upconversion Luminescent Nanoparticles—Evolving Role in Bioimaging, Biosensing, and Drug Delivery. Materials 2022, 15, 2374. [Google Scholar] [CrossRef]
- Zhang, Q.; O’Brien, S.; Grimm, J. Biomedical Applications of Lanthanide Nanomaterials, for Imaging, Sensing and Therapy. Nanotheranostics 2022, 6, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Hu, C.; Su, Q. Luminescent Lifetime Regulation of Lanthanide-Doped Nanoparticles for Biosensing. Biosensors 2022, 12, 131. [Google Scholar] [CrossRef] [PubMed]
- Francés-Soriano, L.; Ferrera-González, J.; González-Béjar, M.; Pérez-Prieto, J. Near-Infrared Excitation/Emission Microscopy with Lanthanide-Based Nanoparticles. Anal. Bioanal. Chem. 2022, 414, 4291–4310. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Jiang, M.; Zeng, S.; Liu, H. Polydopamine coated multifunctional lanthanide theranostic agent for vascular malformation and tumor vessel imaging beyond 1500 nm and imaging-guided photothermal therapy. Theranostics 2019, 9, 3866–3878. [Google Scholar] [CrossRef]
- Zhou, T.; Cheng, Q.; Zhang, L.; Zhang, D.; Li, L.; Jiang, T.; Huang, L.; Xu, H.; Hu, M.; Jing, S. Ferrocene-functionalized core–shell lanthanide-doped upconversion nanoparticles: NIR light promoted chemodynamic therapy and luminescence imaging of solid tumors. Chem. Eng. J. 2022, 438, 135637. [Google Scholar] [CrossRef]
- Silvestri, B.; Armanetti, P.; Sanità, G.; Vitiello, G.; Lamberti, A.; Calì, G.; Pezzella, A.; Luciani, G.; Menichetti, L.; Luin, S.; et al. Silver-nanoparticles as plasmon-resonant enhancers for eumelanin′s photoacoustic signal in a self-structured hybrid nanoprobe. Mater. Sci. Eng. C 2019, 102, 788–797. [Google Scholar] [CrossRef]
- Carrese, B.; Cavallini, C.; Sanità, G.; Armanetti, P.; Silvestri, B.; Calì, G.; Pota, G.; Luciani, G.; Menichetti, L.; Lamberti, A. Controlled Release of Doxorubicin for Targeted Chemo-Photothermal Therapy in Breast Cancer HS578T Cells Using Albumin Modified Hybrid Nanocarriers. Int. J. Mol. Sci. 2021, 22, 11228. [Google Scholar] [CrossRef]
- Ferreira, C.A.; Goel, S.; Ehlerding, E.B.; Rosenkrans, Z.T.; Jiang, D.; Sun, T.; Aluicio-Sarduy, E.; Engle, J.W.; Ni, D.; Cai, W. Ultrasmall Porous Silica Nanoparticles with Enhanced Pharmacokinetics for Cancer Theranostics. Nano Lett. 2021, 21, 4692–4699. [Google Scholar] [CrossRef]
- Nikdouz, A.; Namarvari, N.; Shayan, R.G.; Hosseini, A. Comprehensive Comparison of Theranostic Nanoparticles in Breast Cancer. Am. J. Clin. Exp. Immunol. 2022, 11, 1–27. [Google Scholar]
- Calzoni, E.; Cesaretti, A.; Polchi, A.; Di Michele, A.; Tancini, B.; Emiliani, C. Biocompatible Polymer Nanoparticles for Drug Delivery Applications in Cancer and Neurodegenerative Disorder Therapies. J. Funct. Biomater. 2019, 10, 4. [Google Scholar] [CrossRef]
- Zielińska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Venkatesh, D.N.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef] [PubMed]
- Parveen, S.; Sahoo, S.K. Polymeric nanoparticles for cancer therapy. J. Drug Target. 2008, 16, 108–123. [Google Scholar] [CrossRef] [PubMed]
- Muthu, M.S.; Leong, D.T.; Mei, L.; Feng, S.-S. Nanotheranostics—Application and Further Development of Nanomedicine Strategies for Advanced Theranostics. Theranostics 2014, 4, 660–677. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Li, D.; Hao, Y.; Niu, M.; Hu, Y.; Zhao, H.; Chang, J.; Zhang, Z.; Zhang, Y. Gold nanorod–based poly(lactic-co-glycolic acid) with manganese dioxide core–shell structured multifunctional nanoplatform for cancer theranostic applications. Int. J. Nanomed. 2017, 12, 3059–3075. [Google Scholar] [CrossRef] [PubMed]
- Asati, S.; Pandey, V.; Soni, V. RGD Peptide as a Targeting Moiety for Theranostic Purpose: An Update Study. Int. J. Pept. Res. Ther. 2019, 25, 49–65. [Google Scholar] [CrossRef]
- Dong, Q.; Yang, H.; Wan, C.; Zheng, D.; Zhou, Z.; Xie, S.; Xu, L.; Du, J.; Li, F. Her2-Functionalized Gold-Nanoshelled Magnetic Hybrid Nanoparticles: A Theranostic Agent for Dual-Modal Imaging and Photothermal Therapy of Breast Cancer. Nanoscale Res. Lett. 2019, 14, 235. [Google Scholar] [CrossRef]
- Gholami, L.; Tafaghodi, M.; Abbasi, B.; Daroudi, M.; Kazemi Oskuee, R. Preparation of superparamagnetic iron oxide/doxorubicin loaded chitosan nanoparticles as a promising glioblastoma theranostic tool. J. Cell. Physiol. 2019, 234, 1547–1559. [Google Scholar] [CrossRef]
- Sahoo, A.K.; Banerjee, S.; Ghosh, S.S.; Chattopadhyay, A. Simultaneous RGB Emitting Au Nanoclusters in Chitosan Nanoparticles for Anticancer Gene Theranostics. ACS Appl. Mater. Interfaces 2014, 6, 712–724. [Google Scholar] [CrossRef]
- Stanley, S. Biological nanoparticles and their influence on organisms. Curr. Opin. Biotechnol. 2014, 28, 69–74. [Google Scholar] [CrossRef]
- Luk, B.T.; Fang, R.H.; Zhang, L. Lipid- and Polymer-Based Nanostructures for Cancer Theranostics. Theranostics 2012, 2, 1117–1126. [Google Scholar] [CrossRef]
- Feng, L.; Cheng, L.; Dong, Z.; Tao, D.; Barnhart, T.E.; Cai, W.; Chen, M.; Liu, Z. Theranostic Liposomes with Hypoxia-Activated Prodrug to Effectively Destruct Hypoxic Tumors Post-Photodynamic Therapy. ACS Nano 2017, 11, 927–937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, R.; Yadav, A.S.; Gorain, M.; Chauhan, D.S.; Kundu, G.C.; Srivastava, R.; Selvaraj, K. Graphene Oxide Supported Liposomes as Red Emissive Theranostics for Phototriggered Tissue Visualization and Tumor Regression. ACS Appl. Bio Mater. 2019, 2, 3312–3320. [Google Scholar] [CrossRef] [PubMed]
- Park, C.R.; Jo, J.H.; Song, M.G.; Park, J.Y.; Kim, Y.-H.; Youn, H.; Paek, S.H.; Chung, J.-K.; Jeong, J.M.; Lee, Y.-S.; et al. Secreted protein acidic and rich in cysteine mediates active targeting of human serum albumin in U87MG xenograft mouse models. Theranostics 2019, 9, 7447–7457. [Google Scholar] [CrossRef]
- Zhao, W.; Li, T.; Long, Y.; Guo, R.; Sheng, Q.; Lu, Z.; Li, M.; Li, J.; Zang, S.; Zhang, Z.; et al. Self-promoted Albumin-Based Nanoparticles for Combination Therapy against Metastatic Breast Cancer via a Hyperthermia-Induced “Platelet Bridge”. ACS Appl. Mater. Interfaces 2021, 13, 25701–25714. [Google Scholar] [CrossRef]
- Sun, X.; Cui, Z. Virus-Like Particles as Theranostic Platforms. Adv. Ther. 2020, 3, 1900194. [Google Scholar] [CrossRef]
- Chung, Y.H.; Cai, H.; Steinmetz, N.F. Viral nanoparticles for drug delivery, imaging, immunotherapy, and theranostic applications. Adv. Drug Deliv. Rev. 2020, 156, 214–235. [Google Scholar] [CrossRef]
- Pitek, A.S.; Hu, H.; Shukla, S.; Steinmetz, N.F. Cancer Theranostic Applications of Albumin-Coated Tobacco Mosaic Virus Nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 39468–39477. [Google Scholar] [CrossRef]
- Yan, J.; Yu, J.; Wang, C.; Gu, Z. Red Blood Cells for Drug Delivery. Small Methods 2017, 1, 1700270. [Google Scholar] [CrossRef]
- Guido, C.; Maiorano, G.; Gutiérrez-Millán, C.; Cortese, B.; Trapani, A.; D’Amone, S.; Gigli, G.; Palamà, I. Erythrocytes and Nanoparticles: New Therapeutic Systems. Appl. Sci. 2021, 11, 2173. [Google Scholar] [CrossRef]
- Wang, C.; Sun, X.; Cheng, L.; Yin, S.; Yang, G.; Li, Y.; Liu, Z. Multifunctional Theranostic Red Blood Cells for Magnetic-Field-Enhanced in vivo Combination Therapy of Cancer. Adv. Mater. 2014, 26, 4794–4802. [Google Scholar] [CrossRef]
- Burns, J.M.; Vankayala, R.; Mac, J.T.; Anvari, B. Erythrocyte-Derived Theranostic Nanoplatforms for Near Infrared Fluorescence Imaging and Photodestruction of Tumors. ACS Appl. Mater. Interfaces 2018, 10, 27621–27630. [Google Scholar] [CrossRef] [PubMed]




| Inorganic NPs | |||||
|---|---|---|---|---|---|
| Size and Superficial Charge | Diagnosis | Therapy | Model | Ref. | |
| Iron Oxide NPs | Size 45.7 nm ζ-potential n.r. | Magnetic Resonance Imaging | Magnetic resonance-guided focused ultrasound surgery | In vitro H460 cells In vivo H460 xenograft mice | [37] |
| Iron Oxide NPs | Size 50.8 nm ± 5.2 ζ-potential n.r. | Magnetic Resonance Imaging | Doxorubicin | In vitro 4T1 cells In vivo 4T1 xenograft mice | [38] |
| Iridium oxide NPs | Size 55.0 nm ζ-potential −0.40 mV | Fluorescence imaging | Doxorubicin Photothermal Therapy | In vitro HepG2 cells In vivo HepG2 xenograft mice | [40] |
| MOF Fe3O4@UiO-66 | Size 241.5 nm ± 28.5 ζ-potential −25.7 mV ± 5.2 | Magnetic Resonance Imaging | Doxorubicin | In vitro HeLa cells In vivo HeLa-tumor bearing mice | [44] |
| MOF NaGdF4:Yb/Er@MIL-53(Fe) | Size 245 nm ± 5.0 ζ-potential n.r. | Magnetic Resonance Imaging | Doxorubicin | In vitro B16−F10 and HEK293 cells | [45] |
| Gold NPs | Size 26.5 nm ± 1.1 ζ-potential n.r. | Fluorescence imaging | Photodynamic Therapy | In vitro PC-3 cells In vivo PC-3 xenograft mice | [49] |
| Gold NPs | Size 390.0 nm ζ-potential n.r. | Photoacoustic imaging | Photothermal Therapy | In vitro U-87MG cells In vivo U-87MG xenograft mice | [50] |
| Lanthanide-doped NPs NaYF4:Yb, Tm@NaYF4:Eu | Size 141.9 nm ζ-potential −20.2 mV | Upconversion luminescence imaging | Photodynamic Therapy | In vitro AGS cells In vivo AGS xenograft mice | [56] |
| Lanthanide-doped NPs NaLuF4 | Size 20 × 130 nm ζ-potential n.r. | NIR-II imaging | Photothermal therapy | In vitro HeLa cells In vivo HCT 116 xenograft mice and LLC | [55] |
| Silicon-based | Size 407.0 nm ± 29.0 ζ-potential −17.0 mV ± 2.16 | Photoacoustic Imaging | Photothermal therapy Doxorubicin | In vitro MCF10a and HS578T cells | [20,57,58] |
| Silicon-based | Size 13.5 nm ζ-potential n.r. | PET imaging | Radiotherapy | In vivo 4T1 tumor-bearing mice | [59] |
| Organic NPs | |||||
| PLGA-based NPs | Size 282.1 nm ± 6.2 ζ-potential −9.7 mV ± 1.4 | Magnetic Resonance Imaging | Radio frequency hyperthermia Docetaxel | In vitro MCF7 cells In vivo S180 xenograft mice | [65] |
| PLGA-based NPs | Size 185.1 nm ± 3.3 ζ-potential −1.2 mV ± 0.7 | Photoacoustic imaging | Photothermal therapy | In vitro MDA-MB-231 cells In vivo MDA-MB-231 xenograft mice | [26] |
| PLGA-based NPs | Size 248.3 nm ζ-potential −14.7 mV | Magnetic Resonance Imaging Dual-modal ultrasound | Photothermal therapy | In vitro SKBR3 and MDA-MB-231 cells | [67] |
| Chitosan-based NPs | Size 184.3 nm ± 4.4 ζ-potential + 17.33 mV ± 1.5 | Magnetic Resonance Imaging | Doxorubicin | In vitro C6 cells | [68] |
| Chitosan-based NPs | Size 92.2 nm ζ-potential + 24.0 mV | Fluorescence imaging | Nucleic acid | In vitro HeLa cells | [69] |
| Liposomes-based NPs | Size 95.0 nm ζ-potential n.r. | Positron Emission Tomography Fluorescence Photoacoustic imaging | Photodynamic therapy AQ4N | In vivo 4T1 Balb/c mice | [72] |
| Liposomes-based NPs | Size 150–300 nm ζ-potential + 13.2 mV | Fluorescence imaging | Photothermal therapy Doxorubicin | In vitro MDA-MB-231 and 4T1 cells In vivo 4T1 Balb/c mice | [73] |
| Albumin NPs | Size 142.2 nm ± 4.86 ζ-potential −30 mV | Fluorescence imaging | Photodynamic therapy Photothermal therapy Paclitaxel | In vitro 4T1 cells In vivo 4T1 Balb/c mice | [75] |
| Virus like-NPs | Size 212.0 nm ± 3.40 ζ-potential n.r. | Fluorescence imaging | Doxorubicin | In vitro 4T1and MDA-MB-231 cells In vivo 4T1 Balb/c mice MDA-MB-231 and PC-3 xenograft mice | [78] |
| Red Blood cells-based NPs | Size about 7 µm ζ-potential n.r. | Magnetic Resonance Imaging Fluorescence imaging | Photodynamic therapy Doxorubicin | In vitro 4T1 cells In vivo 4T1 Balb/c mice | [81] |
| Red Blood cells-based NPs | Size 79.0 nm ζ-potential n.r. | Fluorescence imaging | Photodestruction | In vitro SKBR3 cells In vivo SKBR3 xenograft mice | [82] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrese, B.; Sanità, G.; Lamberti, A. Nanoparticles Design for Theranostic Approach in Cancer Disease. Cancers 2022, 14, 4654. https://doi.org/10.3390/cancers14194654
Carrese B, Sanità G, Lamberti A. Nanoparticles Design for Theranostic Approach in Cancer Disease. Cancers. 2022; 14(19):4654. https://doi.org/10.3390/cancers14194654
Chicago/Turabian StyleCarrese, Barbara, Gennaro Sanità, and Annalisa Lamberti. 2022. "Nanoparticles Design for Theranostic Approach in Cancer Disease" Cancers 14, no. 19: 4654. https://doi.org/10.3390/cancers14194654