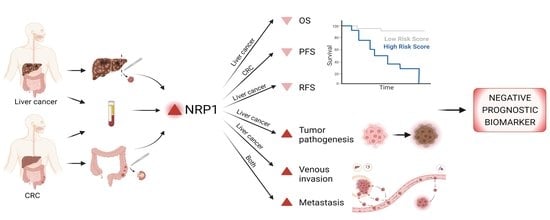
Neuropilin-1 as a Potential Biomarker of Prognosis and Invasive-Related Parameters in Liver and Colorectal Cancer: A Systematic Review and Meta-Analysis of Human Studies
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Objectives
2.2. Literature Search Strategy
2.3. Inclusion and Exclusion Criteria
2.4. Data Extraction and Quality Assessment
2.5. Statistical Analysis
3. Results
3.1. Study Selection and Study Characteristics

| Study | Publication Year | Tumor Type | Tumor Sample Size (M/F) | Non-Tumor Sample Size | Intervention | Pre- or Post-Surgery Treatment | Study Quality (NOS Score) | Measurement of NRP1 Expression | Cut-Off Value for “High” NRP1 Expression | Tumor Samples with “High” NRP1 (%) | Non-Tumor Samples with “High” NRP1 (%) | Parameter Analyzed | HR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Deng et al. [29] | 2021 | HCC, CCA, CRC | 845 (NR) | NA | NR | NR | 5/9 | Tissue levels-RNA-seq | >median | 25.09 (25.20,25.00, 25.00) | NA | OS | Reported |
| Li et al. [32] | 2021 | HCC | 239 (215/24) | 16 | Curative hepatic resection | No | 7/9 | Tissue levels-IHC | >1 a | 57.74 | 37.50 | OS CF Pathogenesis | Estimated |
| Liu et al. [33] | 2021 | CRC (COAD) | 279 (154/125) | NA | NR | NR | 6/9 | Tissue levels-RNA-seq | NR | 25.09 | NA | OS | Estimated |
| Bianconi et al. [28] | 2020 | CRC | 74 (54/20) | NA | Surgery | Yes b | 6/9 | Tissue levels-IHC | >1 c | NR | NA | OS, PFS | Reported |
| Giannelli et al. [30] | 2020 | HCC | 149 (127/22) | NA | No | Yes d | 6/9 | Serum levels | >median | NR | NA | OS | Reported |
| Wu et al. [23] | 2020 | CCA (ICC) | 291 (174/117) | 55 | Surgery | No | 7/9 | Tissue levels-IHC and qRT-PCR e | Strong f | 64.60 | NR | OS, RFS CF | Estimated |
| Zhu et al. [16] | 2020 | CCA | 39 (24/15) | 39 | NR | No | 7/9 | Tissue levels-IHC and qRT-PCR g | >mean | 46.15 | NR | CF | NR |
| Lin et al. [14] | 2018 | HCC | 40 (28/12) | 30 | Surgery | No | 6/9 | Tissue levels-IHC | NR | 72.50 | 0.00 | Pathogenesis | NR |
| Benson et al. [26] | 2016 | CRC | 162 (NR) | NA | No | Yes h | 5/9 | Serum levels | ≥median | 50.62 | NA | PFS | Estimated |
| Zhang et al. [25] | 2016 | HCC | 105 (77/28) | 105 | Surgery | No | 7/9 | Tissue levels-IHC | >3 i | 53.33 | 20.95 | OS, RFS Pathogenesis CF | Estimated |
| Spencer et al. [22] | 2013 | CRC | 583 (NR) | NA | No | Yes j | 5/9 | Serum levels | >median | 48.71 | NA | OS, PFS | Reported |
| Yaqoob et al. [24] | 2012 | HCC | 93 (NR) | NA | Surgery | No | 5/9 | Tissue levels-PCR array | >75% of expression | 63.44 | NA | OS | Estimated |
| Berge et al. [27] | 2011 | HCC | 308 (NR) | 31 | Surgery | No | 6/9 | Tissue levels-IHC | ≥1 k | 50.65 | 0.00 | Pathogenesis | NR |
| Kamiya et al. [31] | 2006 | CRC | 54 (NR) | 54 | Surgery | No | 6/9 | Tissue levels-qRT-PCR | ≥0.5 l | 37.04 for survival-22.22 for CF | 62.96 | OS CF Pathogenesis | Estimated |
| Ochiumi et al. [21] | 2006 | CRC | 103 (NR) for survival-146 (91/55) for CF | NA | Surgery | Yes m | 6/9 | Tissue levels-IHC | SIS+MVS > 3.64 n | 60.19 for survival-65.07 for CF | NA | OS CF | Estimated |
3.2. Overall Survival
3.3. Recurrence-Free Survival and Progression-Free Survival
3.4. Tumor Pathogenesis
3.5. Tumor-Associated Clinicopathological Features
3.6. Meta-Regression
3.7. Subgroup Analysis
3.8. Analysis of Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zygulska, A.L.; Pierzchalski, P. Novel diagnostic biomarkers in colorectal cancer. Int. J. Mol. Sci. 2022, 23, 852. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.Y.; Chen, K.F.; Chen, P.J. Treatment of liver cancer. Cold Spring Harb. Perspect. Med. 2015, 5, a021535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anwanwan, D.; Singh, S.K.; Singh, S.; Saikam, V.; Singh, R. Challenges in liver cancer and possible treatment approaches. Biochim. Biophys. Acta—Rev. Cancer 2020, 1873, 188314. [Google Scholar] [CrossRef] [PubMed]
- Prieto-Domínguez, N.; Méndez-Blanco, C.; Carbajo-Pescador, S.; Fondevila, F.; García-Palomo, A.; González-Gallego, J.; Mauriz, J.L. Melatonin enhances sorafenib actions in human hepatocarcinoma cells by inhibiting mTORC1/p70S6K/HIF-1α and hypoxia mediated mitophagy. Oncotarget 2017, 8, 91402–91414. [Google Scholar] [CrossRef]
- Melli, F.; Bartolini, I.; Risaliti, M.; Tucci, R.; Ringressi, M.N.; Muiesan, P.; Taddei, A.; Amedei, A. Evaluation of prognostic factors and clinicopathological patterns of recurrence after curative surgery for colorectal cancer. World J. Gastrointest. Surg. 2021, 13, 50–75. [Google Scholar] [CrossRef]
- Dumond, A.; Pagès, G. Neuropilins, as relevant oncology target: Their role in the tumoral microenvironment. Front. Cell Dev. Biol. 2020, 8, 662. [Google Scholar] [CrossRef]
- Niland, S.; Eble, J.A. Neuropilins in the context of tumor vasculature. Int. J. Mol. Sci. 2019, 20, 639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Napolitano, V.; Tamagnone, L. Neuropilins controlling cancer therapy responsiveness. Int. J. Mol. Sci. 2019, 20, 2049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, C.; Jiang, X. Role of NRP-1 in VEGF-VEGFR2-independent tumorigenesis. Targ. Oncol. 2016, 11, 501–505. [Google Scholar] [CrossRef]
- Lin, J.; Zhang, Y.; Wu, J.; Li, L.; Chen, N.; Ni, P.; Song, L.; Liu, X. Neuropilin 1 (NRP1) is a novel tumor marker in hepatocellular carcinoma. Clin. Chim. Acta 2018, 485, 158–165. [Google Scholar] [CrossRef]
- Tomida, C.; Yamagishi, N.; Nagano, H.; Uchida, T.; Ohno, A.; Hirasaka, K.; Nikawa, T.; Da Teshima-Kondo, S. VEGF pathway-targeting drugs induce evasive adaptation by activation of neuropilin-1/cMet in colon cancer cells. Int. J. Oncol. 2018, 52, 1350–1362. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Zhai, B.; He, C.; Li, Z.; Gao, H.; Niu, Z.; Jiang, X.; Lu, J.; Sun, X. LncRNA TTN-AS1 promotes the progression of cholangiocarcinoma via the miR-320a/neuropilin-1 axis. Cell Death Dis. 2020, 11, 637. [Google Scholar] [CrossRef]
- Cao, H.; Li, Y.; Huang, L.; Bai, B.; Xu, Z. Clinicopathological significance of Neuropilin 1 expression in gastric cancer: A meta-analysis. Dis. Markers 2020, 2020, 4763492. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brenna, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 3 June 2022).
- Parmar, M.K.B.; Torri, V.; Stewart, L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat. Med. 1998, 17, 2815–2834. [Google Scholar] [CrossRef]
- Ochiumi, T.; Kitadai, Y.; Tanaka, S.; Akagi, M.; Yoshihara, M.; Chayama, K. Neuropilin-1 is involved in regulation of apoptosis and migration of human colon cancer. Int. J. Oncol. 2006, 29, 105–116. [Google Scholar] [CrossRef] [Green Version]
- Spencer, S.K.M.; Pommier, A.J.C.; Morgan, S.R.; Barry, S.T.; Robertson, J.D.; Hoff, P.M.; Jürgensmeier, J.M. Prognostic/predictive value of 207 serum factors in colorectal cancer treated with cediranib and/or chemotherapy. Br. J. Cancer 2013, 109, 2765–2773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.-N.; He, L.-H.; Bai, Z.-T.; Li, X. NRP1 is a prognostic factor and promotes the growth and migration of cells in intrahepatic cholangiocarcinoma. Cancer Manag. Res. 2020, 12, 7021–7032. [Google Scholar] [CrossRef]
- Yaqoob, U.; Cao, S.; Shergill, U.; Jagavelu, K.; Geng, Z.; Yin, M.; de Assuncao, T.M.; Cao, Y.; Szabolcs, A.; Thorgeirsson, S.; et al. Neuropilin-1 stimulates tumor growth by increasing fibronectin fibril assembly in the tumor microenvironment. Cancer Res. 2012, 72, 4047–4059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Liu, P.; Jiang, Y.; Dou, X.; Yan, J.; Ma, C.; Fan, Q.; Wang, W.; Su, F.; Tang, H.; et al. High expression of neuropilin-1 associates with unfavorable clinicopathological features in hepatocellular carcinoma. Pathol. Oncol. Res. 2016, 22, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B.; Kiss, I.; Bridgewater, J.; Eskens, F.A.L.M.; Sasse, C.; Vossen, S.; Chen, J.; Van Sant, C.; Ball, H.A.; Keating, A.; et al. BATON-CRC: A phase II randomized trial comparing tivozanib plus mFOLFOX6 with bevacizumab plus mFOLFOX6 in stage IV metastatic colorectal cancer. Clin. Cancer Res. 2016, 22, 5058–5067. [Google Scholar] [CrossRef] [Green Version]
- Bergé, M.; Allanic, D.; Bonnin, P.; De Montrion, C.; Richard, J.; Suc, M.; Boivin, J.-F.; Contrers, J.-O.; Lockhart, B.P.; Pocard, M.; et al. Neuropilin-1 is upregulated in hepatocellular carcinoma and contributes to tumour growth and vascular remodelling. J. Hepatol. 2011, 55, 866–875. [Google Scholar] [CrossRef]
- Bianconi, D.; Herac, M.; Posch, F.; Schmeidl, M.; Unseld, M.; Kieler, M.; Brettner, R.; Müllauer, L.; Riedl, J.; Gerger, A.; et al. Microvascular density assessed by CD31 predicts clinical benefit upon bevacizumab treatment in metastatic colorectal cancer: Results of the PassionATE study, a translational prospective Phase II study of capecitabine and irinotecan plus bevacizumab follow. Ther. Adv. Med. Oncol. 2020, 12, 1758835920928635. [Google Scholar] [CrossRef]
- Deng, C.; Guo, H.; Yan, D.; Liang, T.; Ye, X.; Li, Z. Pancancer analysis of neurovascular-related NRP family genes as potential prognostic biomarkers of bladder urothelial carcinoma. BioMed Res. Int. 2021, 2021, 5546612. [Google Scholar] [CrossRef]
- Giannelli, G.; Santoro, A.; Kelley, R.K.; Gane, E.; Paradis, V.; Cleverly, A.; Smith, C.; Estrem, S.T.; Man, M.; Wang, S.; et al. Biomarkers and overall survival in patients with advanced hepatocellular carcinoma treated with TGF-βRI inhibitor galunisertib. PLoS ONE 2020, 15, e0222259. [Google Scholar] [CrossRef] [Green Version]
- Kamiya, T.; Kawakami, T.; Abe, Y.; Nishi, M.; Onoda, N.; Miyazaki, N.; Oida, Y.; Yamazaki, H.; Ueyama, Y.; Nakamura, M. The preserved expression of neuropilin (NRP) 1 contributes to a better prognosis in colon cancer. Oncol. Rep. 2006, 15, 369–373. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zhou, Y.; Hu, J.; Bai, Z.; Meng, W.; Zhang, L.; Song, X.; Wei, Y.; Yan, J.; Zhou, Y. Loss of neuropilin1 inhibits liver cancer stem cells population and blocks metastasis in hepatocellular carcinoma via epithelial-mesenchymal transition. Neoplasma 2021, 68, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Meng, X.; Peng, X.; Yao, Q.; Zhu, F.; Ding, Z.; Sun, H.; Liu, X.; Li, D.; Lu, Y.; et al. Impaired AGO2/miR-185-3p/NRP1 axis promotes colorectal cancer metastasis. Cell Death Dis. 2021, 12, 390. [Google Scholar] [CrossRef] [PubMed]
- Harada, S.; Morlote, D. Molecular pathology of colorectal cancer. Adv. Anat. Pathol. 2020, 27, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Yiu, A.J.; Yiu, C.Y. Biomarkers in colorectal cancer. Anticancer Res. 2016, 36, 1093–1338. [Google Scholar] [PubMed]
- Méndez-Blanco, C.; Fernández-Palanca, P.; Fondevila, F.; González-Gallego, J.; Mauriz, J.L. Prognostic and clinicopathological significance of hypoxia-inducible factors 1α and 2α in hepatocellular carcinoma: A systematic review with meta-analysis. Ther. Adv. Med. Oncol. 2021, 13, 1758835920987071. [Google Scholar] [CrossRef]
- Moloudizargari, M.; Rahmani, J.; Asghari, M.H.; Goel, A. The prognostic role of miR-31 in colorectal cancer: The results of a meta-analysis of 4720 patients. Epigenomics 2022, 14, 101–112. [Google Scholar] [CrossRef]
- Fondevila, F.; Fernández-Palanca, P.; Méndez-Blanco, C.; Payo-Serafín, T.; Lozano, E.; Marin, J.J.G.; González-Gallego, J.; Mauriz, J.L. Association of FOXO3 expression with tumor pathogenesis, prognosis and clinicopathological features in hepatocellular carcinoma: A systematic review with meta-analysis. Cancers 2021, 13, 5349. [Google Scholar] [CrossRef]
- Zhuo, Y.J.; Shi, Y.; Wu, T. NRP-1 and kdr polymorphisms are associated with survival time in patients with advanced gastric cancer. Oncol. Lett. 2019, 18, 4629–4638. [Google Scholar] [CrossRef]
- Jin, Q.; Ren, Q.; Chang, X.; Yu, H.; Jin, X.; Lu, X.; He, N.; Wang, G. Neuropilin-1 predicts poor prognosis and promotes tumor metastasis through epithelial-mesenchymal transition in gastric cancer. J. Cancer 2021, 12, 3648–3659. [Google Scholar] [CrossRef]
- Ben, Q.; Zheng, J.; Fei, J.; An, W.; Li, P.; Li, Z.; Yuan, Y. High neuropilin 1 expression was associated with angiogenesis and poor overall survival in resected pancreatic ductal adenocarcinoma. Pancreas 2014, 43, 744–749. [Google Scholar] [CrossRef]
- Yang, L.; Liu, L.; Zhu, Y.; Wang, B.; Chen, Y.; Zhang, F.; Zhang, X.-A.; Ren, C.-C. Neuropilin-1 is associated with the prognosis of cervical cancer in Henan Chinese population. Onco. Targets. Ther. 2019, 12, 2911–2920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siamakpour-Reihani, S.; Owzar, K.; Jiang, C.; Turner, T.; Deng, Y.; Bean, S.M.; Horton, J.K.; Berchuck, A.; Marks, J.R.; Dewhirst, M.W.; et al. Prognostic significance of differential expression of angiogenic genes in women with high-grade serous ovarian carcinoma. Physiol. Behav. 2015, 139, 23–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Zeheimi, N.; Naik, A.; Bakheit, C.S.; Al Riyami, M.; Al Ajarrah, A.; Al Badi, S.; Al Baimani, K.; Malik, K.; Al Habsi, Z.; Al Moundhri, M.S.; et al. Neoadjuvant chemotherapy alters neuropilin-1, PlGF, and SNAI1 expression levels and predicts breast cancer patients response. Front. Oncol. 2019, 9, 323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, M.; Liu, L.; Hu, C.; Liu, Y.; Qiao, Y.; Jiang, X. Expression of VEGFR2 and NRP-1 in non-small cell lung cancer and their clinical significance. Chinese J. Cancer Res. 2014, 26, 669–677. [Google Scholar] [CrossRef]
- Cheng, W.; Fu, D.; Wei, Z.F.; Xu, F.; Xu, X.F.; Liu, Y.H.; Ge, J.P.; Tian, F.; Han, C.H.; Zhang, Z.Y.; et al. NRP-1 expression in bladder cancer and its implications for tumor progression. Tumor Biol. 2014, 35, 6089–6094. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Cai, H.; Tang, M.; Tang, J. Neuropilin-1 is overexpressed in osteosarcoma and contributes to tumor progression and poor prognosis. Clin. Transl. Oncol. 2014, 16, 732–738. [Google Scholar] [CrossRef]
- Xu, Y.; Li, P.; Zhang, X.; Wang, J.; Gu, D.; Wang, Y. Prognostic implication of neuropilin-1 upregulation in human nasopharyngeal carcinoma. Diagn. Pathol. 2013, 8, 155. [Google Scholar] [CrossRef] [Green Version]
- Valiulyte, I.; Steponaitis, G.; Kardonaite, D.; Tamasauskas, A.; Kazlauskas, A. A SEMA3 signaling pathway-based multi-biomarker for prediction of glioma patient survival. Int. J. Mol. Sci. 2020, 21, 7396. [Google Scholar] [CrossRef]
- Zhuang, P.-Y.; Wang, J.-D.; Tang, Z.-H.; Zhou, X.-P.; Yang, Y.; Quan, Z.-W.; Liu, Y.-B.; Shen, J. Peritumoral Neuropilin-1 and VEGF receptor-2 expression increases time to recurrence in hepatocellular carcinoma patients undergoing curative hepatectomy. Oncotarget 2014, 5, 11121–11132. [Google Scholar] [CrossRef] [Green Version]
- Van Cutsem, E.; De Haas, S.; Kang, Y.K.; Ohtsu, A.; Tebbutt, N.C.; Xu, J.M.; Yong, W.P.; Langer, B.; Delmar, P.; Scherer, S.J.; et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: A biomarker evaluation from the AVAGAST randomized phase III trial. J. Clin. Oncol. 2012, 30, 2119–2127. [Google Scholar] [CrossRef]
- Yoshida, T.; Ohe, C.; Ikeda, J.; Atsumi, N.; Saito, R.; Taniguchi, H.; Ohsugi, H.; Sugi, M.; Tsuta, K.; Matsuda, T.; et al. Integration of NRP1, RGS5, and FOXM1 expression, and tumour necrosis, as a postoperative prognostic classifier based on molecular subtypes of clear cell renal cell carcinoma. J. Pathol. Clin. Res. 2021, 7, 590–603. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.M.; Chen, Y.L.; Wu, Y.Y.; Yuan, A.; Chao, Y.C.; Chung, Y.C.; Wu, M.H.; Yang, S.C.; Pan, S.H.; Shih, J.Y.; et al. Targeting neuropilin 1 as an antitumor strategy in lung cancer. Clin. Cancer Res. 2007, 13, 4759–4768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomida, C.; Yamagishi, N.; Nagano, H.; Uchida, T.; Ohno, A.; Hirasaka, K.; Nikawa, T.; Teshima-Kondo, S. Antiangiogenic agent sunitinib induces epithelial to mesenchymal transition and accelerates motility of colorectal cancer cells. J. Med. Investig. 2017, 64, 250–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.W.; Lien, J.C.; Kuo, S.C.; Huang, T.F. DDA suppresses angiogenesis and tumor growth of colorectal cancer in vivo through decreasing VEGFR2 signaling. Oncotarget 2016, 7, 63124–63137. [Google Scholar] [CrossRef] [Green Version]




| Variable | Beta Coefficient | z | p-Value | 95% CI | Residual Heterogeneity | ||
|---|---|---|---|---|---|---|---|
| I2 | Q Test p-Value | R2 | |||||
| OS | |||||||
| Sample size | 1.00 | 0.66 | 0.51 | 0.999–1.001 | 99.68% | 0.00 | 0.00% |
| Follow-up | 1.00 | −0.96 | 0.34 | 0.992–1.003 | 99.74% | 0.00 | 2.26% |
| NOS | 1.28 | 2.32 | 0.02 | 1.038–1.570 | 99.68% | 0.00 | 42.46% |
| OS, CRC | |||||||
| Sample size | 1.00 | 2.17 | 0.03 | 1.000–1.002 | 92.07% | 0.00 | 59.33% |
| Follow-up | 1.00 | −0.60 | 0.55 | 0.993–1.004 | 98.69% | 0.00 | 0.00% |
| NOS | 1.03 | 0.11 | 0.91 | 0.591–1.802 | 99.08% | 0.00 | 0.00% |
| OS, Liver cancer | |||||||
| Sample size | 1.00 | −0.20 | 0.84 | 0.997–1.003 | 85.16% | 0.00 | 0.00% |
| Follow-up | 1.00 | −0.27 | 0.78 | 0.987–1.010 | 86.76% | 0.00 | 0.00% |
| NOS | 1.32 | 2.36 | 0.02 | 1.048–1.665 | 99.61% | 0.03 | 61.08% |
| PFS | |||||||
| Sample size | 1.00 | −0.14 | 0.89 | 0.997–1.002 | 59.73% | 0.07 | 0.00% |
| Follow-up | 0.98 | −0.94 | 0.35 | 0.941–1.022 | 45.56% | 0.15 | 14.88% |
| NOS | 0.63 | −0.59 | 0.55 | 0.135–2.921 | 68.88% | 0.05 | 0.00% |
| Tumor tissue vs. Adjacent tissue | |||||||
| Sample size | 1.00 | 0.45 | 0.65 | 0.983–1.028 | 94.94% | 0.00 | 0.00% |
| NOS | 0.32 | −0.46 | 0.64 | 0.002–41.134 | 91.57% | 0.00 | 0.00% |
| A. OS | |||||||||
| Subgroup | Studies (n) | Cases (n) | Pooled HR | Test for Heterogeneity | Model Used | ||||
| HR | 95% CI | p- Value | I2 | Q Test p-Value | |||||
| Tumor type | |||||||||
| CRC | 7 | 1537 | 1.22 | 0.96–1.54 | 0.11 | 98.52% | 0.00 | REM | |
| Liver cancer | 7 | 1278 | 1.62 | 1.18–2.21 | 0.00 * | 99.82% | 0.00 | REM | |
| Tumor type | |||||||||
| CCA | 2 | 327 | 1.39 | 0.71–2.74 | 0.34 | 93.68% | 0.00 | REM | |
| CRC | 7 | 1537 | 1.22 | 0.96–1.54 | 0.11 | 98.52% | 0.00 | REM | |
| HCC | 5 | 951 | 1.75 | 1.20–2.56 | 0.00 * | 82.97% | 0.00 | REM | |
| Sample size | |||||||||
| ≤100 | 4 | 257 | 1.01 | 0.99–1.02 | 0.41 | 0.01% | 0.03 | REM | |
| >100 | 10 | 2558 | 1.52 | 1.21–1.91 | 0.00 * | 99.78% | 0.00 | REM | |
| ≤200 | 8 | 779 | 1.36 | 0.98–1.88 | 0.06 | 99.14% | 0.00 | REM | |
| >200 | 6 | 2036 | 1.46 | 1.11–1.93 | 0.01 * | 99.85% | 0.00 | REM | |
| ≤300 | 12 | 1867 | 1.42 | 1.13–1.80 | 0.00 * | 99.58% | 0.00 | REM | |
| >300 | 2 | 948 | 1.32 | 0.76–2.29 | 0.32 | 96.26% | 0.00 | REM | |
| ≤400 | 13 | 2232 | 1.37 | 1.11–1.70 | 0.00 * | 99.82% | 0.00 | REM | |
| >400 | 1 | 583 | 1.77 | 1.43–2.19 | — | — | — | — | |
| NOS scale | |||||||||
| 5 | 6 | 1521 | 1.17 | 0.94–1.45 | 0.15 | 99.86% | 0.00 | REM | |
| 6 | 5 | 659 | 1.45 | 0.90–2.36 | 0.13 | 83.08% | 0.00 | REM | |
| 7 | 3 | 635 | 1.95 | 1.55–2.44 | 0.00 * | 0.00% | 0.81 | FEM † | |
| NOS scale (threshold 5) | |||||||||
| ≤5 | 6 | 1521 | 1.17 | 0.94–1.45 | 0.15 | 99.86% | 0.00 | REM | |
| >5 | 8 | 1294 | 1.64 | 1.25–2.16 | 0.00 * | 72.52% | 0.00 | REM | |
| NOS scale (threshold 6) | |||||||||
| ≤6 | 11 | 2180 | 1.28 | 1.03–1.60 | 0.03 * | 99.83% | 0.00 | REM | |
| >6 | 3 | 635 | 1.95 | 1.55–2.44 | 0.00 * | 0.00% | 0.81 | FEM † | |
| Follow up (months) | |||||||||
| ≤60 | 3 | 806 | 1.62 | 1.01–2.60 | 0.04 * | 91.42% | 0.00 | REM | |
| >60 | 11 | 2009 | 1.34 | 1.06–1.68 | 0.01 * | 99.84% | 0.00 | REM | |
| ≤120 | 8 | 1842 | 1.49 | 1.15–1.92 | 0.00 * | 99.75% | 0.00 | REM | |
| >120 | 6 | 973 | 1.27 | 0.89–1.81 | 0.19 | 99.26% | 0.00 | REM | |
| B. OS in CRC studies | |||||||||
| Subgroup | Studies (n) | Cases (n) | Pooled HR | Test for heterogeneity | Model used | ||||
| HR | 95% CI | p- value | I2 | Q test p-value | |||||
| Sample size | |||||||||
| ≤100 | 2 | 128 | 0.86 | 0.44–1.67 | 0.66 | 56.20% | 0.13 | REM | |
| >100 | 5 | 1409 | 1.34 | 1.00–1.81 | 0.054 | 99.15% | 0.00 | REM | |
| ≤200 | 4 | 396 | 1.02 | 0.99–1.06 | 0.21 | 48.01% | 0.12 | FEM † | |
| >200 | 3 | 1141 | 1.40 | 0.96–2.04 | 0.08 | 91.32% | 0.00 | REM | |
| ≤300 | 6 | 954 | 1.01 | 1.00–1.02 | 0.09 | 0.00% | 0.03 | REM | |
| >300 | 1 | 583 | 1.77 | 1.43–2.19 | — | — | — | — | |
| NOS scale | |||||||||
| 5 | 3 | 1027 | 1.20 | 0.85–1.71 | 0.30 | 99.58% | 0.00 | REM | |
| 6 | 4 | 510 | 1.24 | 0.77–2.00 | 0.37 | 74.05% | 0.02 | REM | |
| Follow up (months) | |||||||||
| ≤120 | 2 | 657 | 1.37 | 0.84–2.24 | 0.21 | 92.86% | 0.00 | REM | |
| >120 | 5 | 880 | 1.13 | 0.87–1.47 | 0.37 | 98.57% | 0.02 | REM | |
| Without Spencer et al. 2013 and Liu et al. 2021 | |||||||||
| 5 | 675 | 1.01 | 1.00–1.02 | 0.10 | 36.07% | 0.18 | FEM † | ||
| C. OS in liver cancer studies | |||||||||
| Subgroup | Studies (n) | Cases (n) | Pooled HR | Test for heterogeneity | Model used | ||||
| HR | 95% CI | p- value | I2 | Q test p-value | |||||
| Sample size | |||||||||
| ≤100 | 2 | 129 | 1.47 | 0.61–3.56 | 0.39 | 83.51% | 0.01 | REM | |
| >100 | 5 | 1149 | 1.71 | 1.21–2.42 | 0.00 * | 85.26% | 0.00 | REM | |
| ≤200 | 4 | 383 | 1.70 | 1.07–2.71 | 0.03 * | 84.83% | 0.00 | REM | |
| >200 | 3 | 895 | 1.56 | 0.96–2.54 | 0.08 | 89.89% | 0.00 | REM | |
| ≤300 | 6 | 913 | 1.78 | 1.29–2.45 | 0.00 * | 82.71% | 0.00 | REM | |
| >300 | 1 | 365 | 1.01 | 1.00–1.02 | — | — | — | — | |
| NOS scale | |||||||||
| 5 | 3 | 494 | 1.18 | 0.80–1.74 | 0.41 | 99.91% | 0.05 | REM | |
| 6 | 1 | 149 | 2.47 | 1.60–3.81 | — | — | — | — | |
| 7 | 3 | 635 | 1.95 | 1.55–2.44 | 0.00 * | 0.00% | 0.81 | FEM † | |
| NOS scale (threshold 5) | |||||||||
| ≤5 | 3 | 494 | 1.18 | 0.80–1.74 | 0.41 | 99.91% | 0.05 | REM | |
| >5 | 4 | 784 | 2.05 | 1.67–2.50 | 0.00 * | 0.00% | 0.72 | FEM † | |
| NOS scale (threshold 6) | |||||||||
| ≤6 | 4 | 643 | 1.47 | 0.89–2.43 | 0.13 | 99.94% | 0.00 | REM | |
| >6 | 3 | 635 | 1.95 | 1.55–2.44 | 0.00 * | 0.00% | 0.81 | FEM † | |
| Follow up (months) | |||||||||
| ≤60 | 1 | 149 | 2.47 | 1.60–3.81 | — | — | — | — | |
| >60 | 6 | 1129 | 1.51 | 1.09–2.08 | 0.01 * | 99.83% | 0.00 | REM | |
| ≤120 | 6 | 1185 | 1.55 | 1.12–2.15 | 0.01 * | 99.85% | 0.00 | REM | |
| >120 | 1 | 93 | 2.51 | 1.21–5.18 | — | — | — | — | |
| D. PFS | |||||||||
| Subgroup | Studies (n) | Cases (n) | Pooled HR | Test for heterogeneity | Model used | ||||
| HR | 95% CI | p- value | I2 | Q test p-value | |||||
| Sample size | |||||||||
| ≤100 | 2 | 128 | 0.98 | 0.47–2.04 | 0.95 | 0.00% | 0.97 | FEM † | |
| >100 | 2 | 691 | 1.86 | 0.85–4.10 | 0.12 | 82.09% | 0.02 | REM | |
| ≤200 | 3 | 236 | 1.56 | 0.70–3.49 | 0.28 | 58.06% | 0.09 | REM | |
| >200 | 1 | 583 | 1.32 | 1.11–1.57 | — | — | — | — | |
| NOS scale | |||||||||
| 5 | 3 | 745 | 1.59 | 0.88–2.86 | 0.12 | 68.88% | 0.05 | REM | |
| 6 | 1 | 74 | 1 | 0.32–3.14 | — | — | — | — | |
| Without Benson et al. 2016 (Tivozanib/mFOLFOX6 group) | |||||||||
| 3 | 711 | 1.3 | 1.10–1.54 | 0.00 * | 0.00% | 0.74 | FEM † | ||
| E. Tumor tissue vs. Adjacent tissue | |||||||||
| Subgroup | Studies (n) | Cases (n) | Cases with high NRP1 expression (%) | Pooled OR | Test for heterogeneity | Model used | |||
| OR | 95% CI | p- value | I2 | Q test p-value | |||||
| Sample size | |||||||||
| ≤100 | 2 | 94 | 88.30 | 6.25 | 0.02–2479.82 | 0.55 | 93.81% | 0.00 | REM |
| >100 | 3 | 652 | 38.34 | 4.48 | 2.59–7.75 | 0.00 * | 49.09% | 0.14 | FEM † |
| ≤200 | 3 | 199 | 80.90 | 4.87 | 0.18–130.34 | 0.34 | 97.06% | 0.00 | REM |
| >200 | 2 | 547 | 31.44 | 10.54 | 0.50–222.18 | 0.13 | 73.98% | 0.05 | REM |
| ≤300 | 4 | 438 | 40.41 | 3.85 | 0.42–35.34 | 0.23 | 94.33% | 0.00 | REM |
| >300 | 1 | 308 | 50.65 | 64.65 | 3.92–1065.96 | — | — | — | — |
| NOS scale | |||||||||
| 6 | 3 | 402 | 59.45 | 12.25 | 0.24–619.71 | 0.21 | 90.15% | 0.00 | REM |
| 7 | 2 | 344 | 27.33 | 4.03 | 2.31–7.05 | 0.00 * | 0.00% | 0.58 | FEM † |
| Survival Parameter | Studies (n) | Egger’s Test (p-Value) | Model Used | Trim-and-Fill Analysis | Studies Imputed (n) | |
| HR | 95% CI | |||||
| OS | 14 | 0.00 * | REM | 1.37 | 1.13–1.68 | 1 |
| PFS | 4 | 0.74 | REM | — | — | — |
| RFS | 2 | 0.40 | FEM | — | — | — |
| OS for CRC | 7 | 0.57 | REM | — | — | — |
| OS for liver cancer | 7 | 0.00* | REM | 1.62 | 1.18–2.21 | 0 |
| Clinicopathological feature | Studies (n) | Egger’s test (p-value) | Model used | Trim-and-fill analysis | Studies imputed (n) | |
| HR | 95% CI | |||||
| Tumor tissue vs. Adjacent tissue | 5 | 0.01 * | REM | 6.19 | 0.77–49.59 | 0 |
| AFP | 2 | 0.96 | FEM | — | — | — |
| Age | 3 | 0.38 | FEM | — | — | — |
| Gender | 4 | 0.87 | FEM | — | — | — |
| Invasion | 3 | 0.24 | FEM | — | — | — |
| Metastasis | 6 | 0.44 | FEM | — | — | — |
| Tumor number | 2 | 0.75 | FEM | — | — | — |
| Tumor size | 5 | 0.68 | FEM | — | — | — |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Palanca, P.; Payo-Serafín, T.; Fondevila, F.; Méndez-Blanco, C.; San-Miguel, B.; Romero, M.R.; Tuñón, M.J.; Marin, J.J.G.; González-Gallego, J.; Mauriz, J.L. Neuropilin-1 as a Potential Biomarker of Prognosis and Invasive-Related Parameters in Liver and Colorectal Cancer: A Systematic Review and Meta-Analysis of Human Studies. Cancers 2022, 14, 3455. https://doi.org/10.3390/cancers14143455
Fernández-Palanca P, Payo-Serafín T, Fondevila F, Méndez-Blanco C, San-Miguel B, Romero MR, Tuñón MJ, Marin JJG, González-Gallego J, Mauriz JL. Neuropilin-1 as a Potential Biomarker of Prognosis and Invasive-Related Parameters in Liver and Colorectal Cancer: A Systematic Review and Meta-Analysis of Human Studies. Cancers. 2022; 14(14):3455. https://doi.org/10.3390/cancers14143455
Chicago/Turabian StyleFernández-Palanca, Paula, Tania Payo-Serafín, Flavia Fondevila, Carolina Méndez-Blanco, Beatriz San-Miguel, Marta R. Romero, María J. Tuñón, Jose J. G. Marin, Javier González-Gallego, and José L. Mauriz. 2022. "Neuropilin-1 as a Potential Biomarker of Prognosis and Invasive-Related Parameters in Liver and Colorectal Cancer: A Systematic Review and Meta-Analysis of Human Studies" Cancers 14, no. 14: 3455. https://doi.org/10.3390/cancers14143455