1. Introduction
Clear cell renal cell carcinoma (CCRCC) is a very prevalent disease and a clinical problem of major concern in Western countries due to its biological aggressiveness and its well-known resistance to chemotherapy and radiotherapy regimes [
1,
2,
3]. Traditionally, radical surgery coupled with early diagnosis has been the only strategy with a direct impact on patient survival [
4]. CCRCC is a model of hypoxia-related disease.
VHL gene malfunction is detected in the overwhelming majority of the cases, resulting in a pseudo-hypoxic status that promotes angiogenesis [
5]. The implementation of antiangiogenic therapies with tyrosine kinase inhibitors has improved the prognosis of many of these patients [
6,
7]. However, its efficacy is limited due to the development of resistant-to-therapy cell clones [
8].
Immune checkpoint blockade of PD-1 and its ligand PD-L1 have been implemented in advanced lung, renal (CCRCC) and bladder carcinomas, as well as in melanoma, with promising results in several trials [
9,
10]. In CCRCC the immunohistochemical evaluation is selectively performed in the intratumor lymphoid inflammatory infiltrates. However, the patient selection for such a form of therapy is difficult, since this evaluation is subjected to interobserver variability [
11]. In fact, up to 17% of patients with negative immunohistochemistry results do respond to this therapy [
12]. Other important limitations for the development of immune checkpoints inhibitors targeting the PD-1 pathway are that responses rates are low and biomarkers are needed for the prediction of treatment responses [
13,
14].
To overcome the aforementioned difficulties, composite biomarkers have been investigated including tumor mutational burden, profiling of tumor infiltrating lymphocytes, molecular subtypes and the characterization of ligand PD-L2. Distinct tumor microenvironment immune types have been described, mainly based on the level of CD8A and PD-1 expression, with the intention to standardize a more comprehensive score to be used as a prognostic marker [
15]. Combination with other composite biomarkers is currently under investigation [
16]. Another interesting strategy to maximize the clinical benefit and predict treatment toxicity is the characterization of gastrointestinal microbiome [
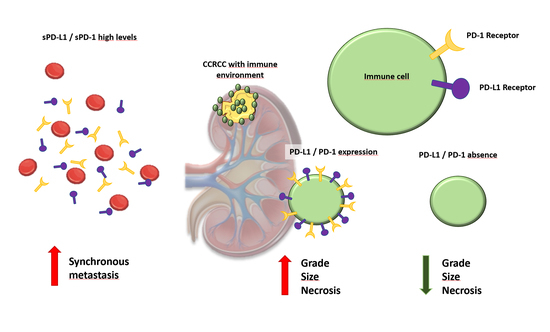
17]. Surprisingly, not much attention has been given to the evaluation of soluble PD-1 (sPD-1) and PD-L1 (sPD-L1) in plasma as potential biomarkers in patients with CCRCC, a heterogeneous neoplasm in serious need of identification of molecular markers that clinicians could use to facilitate an earlier diagnosis, to monitor the disease and to predict prognosis and clinical response to different therapies.
We evaluate plasma and tissue expression of PD-1 and PD-L1 in the same cohort of patients and analyze the relationship between them, also taking into account the non-metastatic and metastatic samples. Within metastatic CCRCC, plasma and tissue expression of PD-1 and PD-L1 were analyzed according to the IMDC risk classification and also according to the Morphology, Attenuation, Size and Structure (MASS) response criteria in patients receiving systemic therapy for metastatic disease. Also, we provide a very interesting simultaneous evaluation of sPD-1 and sPD-L1 and its concomitant expression in the tumor center and infiltrating front, with emphasis on the prognostic implication of these categories. The potential use of sPD-L1 as a tumor marker itself is also discussed, and its relation to other clinical and pathological variables that predict prognosis in CCRCC and treatment response in metastatic CCRCC, according to MASS criteria, is investigated.
3. Discussion
The T-cell coinhibitory receptor programmed death (PD-1) protein and one of its ligands, PD-L1, play an important role in the evasion of the immune system by tumor cells. Both PD-1 and PD-L1 suppress T cell function and immune tolerance [
18]. Recent clinical commercialization of PD-1 pathway inhibitors (nivolumab, pembrolizumab, atezolizumab, durvalumab, avelumab) has raised interest in PD-1 and PD-L1 expression as potential markers of response to immune checkpoint therapy in several malignancies, including CCRCC [
19]. Identification and validation of biomarkers will be crucial to optimize first-line selection of treatment and also treatment sequences.
In this sense, it has been demonstrated that PD-1 and PD-L1 expression is associated with adverse clinico-pathological features in CCRCC, such as a large tumor size, high nuclear grade, tumor necrosis and presence of sarcomatoid differentiation [
20]. What is more, PD-1 expression has been suggested as one of the most interesting biomarkers denoting poor outcomes in patients with metastatic CCRCC receiving molecular targeted therapies, while conflicting results have been shown for PD-L1 in the same population [
20,
21]. Both PD-1 and PD-L1 are expressed in intra-tumor inflammatory lymphocytes [
22]. PD-1 and PD-L1 expression associates with CD4+, CD8+ and FOXP3+ tumor infiltrating lymphocytes related to poor survival in CCRCC [
20,
23,
24]. However, the identification of patients that are likely to obtain a benefit from PD-1/PD-L1 inhibition therapy remains a challenge [
25].
Metastatic CCRCC with a long-term response to sunitinib has been characterized as a distinct phenotype independently associated with low PD-L1 expression [
26]. However, the inherent heterogeneity of CCRCC includes a very variable expression of positive and negative regions of PD-L1 expression within each tumor [
27]. Also, differential expression of PD-1 and PD-L1 has been confirmed between primary and metastatic sites within the same case [
28,
29,
30]. This conflicting scenario can be worsened as the different expression across primary and metastatic tumor for PD-L1 could be associated with metastatic tumor timing. In fact, larger differences between their primary and metastatic tumor pairs have been detected in synchronous metastatic patients in comparison to the metachronous metastatic ones, and this could be explained by the fact that distant metachronous metastasis may have evolved independently of the primary tumor [
31].
PD-L1 expression has been used as surrogate marker of response to immune checkpoint inhibitors and, indirectly, as marker of prognosis as well. In fact, despite all the limitations mentioned to evaluate responses to therapy based on PD-1/PD-L1 expression, a tendency towards a higher PD-L1 expression has been confirmed in responders but without a good correlation [
32]. For this reason, PD-L1 assessment is not required so far to initiate immune checkpoint inhibition therapy in patients with CCRCC. On the other hand, strong evidence is accumulating to consider PD-L1 expression as a likely strong prognosticator in patients with CCRCC not only in metastatic cases receiving anti-PD-1 antibodies, but also receiving sunitinib or pazopanib [
33]. In the series that we present here, PDL-1 expression in CCRCC and sPD-L1 levels were predictors of overall survival, and the combination of both tissue expression and plasma levels was an independent predictor of prognosis. It should be stressed that most of the patients in this series were only treated surgically and therefore, we cannot directly infer that PD-L1 (either tissular or plasmatic) is an independent prognostic marker in patients with metastatic CCRCC treated with systemic therapies. Also, as the tissue and plasma samples analyzed in this series belong to the TKI era (checkpoint immune inhibitors were only used in a small number of patients after progression on TKI). Even though the number of patients with metastatic CCRCC in this series is small, we can confirm that PD-L1 expression in tumor center is higher in metastatic patients within the IMDC poor prognosis group (
p = 0.056) and also that sPD-L1 levels better discriminate poor prognosis for this population of (
p = 0.021).
Circulating sPD-L1 can be determined by ELISA in normal human serum and in supernatants of different cells including CD4+, CD8+, CD19+, CD14+ and CD56+ T cells, and may play an important role in immunoregulation [
34]. sPD-L1 have been described in several malignancies including renal cell cancer, pancreatic cancer, rectal cancer, B-cell lymphoma, multiple myeloma and melanoma [
35,
36,
37,
38,
39]. It has been hypothesized that sPD-L1 may act as a paracrine negative immune regulator within the tumor [
40]. However, the sources of sPD-L1 in patients with cancer is unclear, as it may derive from protumor inflammatory responses, antitumor immune-responses and also intrinsic splicing activities in tumor cells. It is also unclear whether sPD-L1 is associated with clinical characteristics such as patient age, sex or treatment response. In our series sPD-L1 is higher in controls than in patients with CCRCC and the level of sPD-L1 in cancer patients is associated with metastatic disease, but not with conventional prognosticators of CCRCC. Interestingly, higher levels of sPD-L1 in CCRCC are an independent predictor of prognosis. Other authors have investigated the role of several immune checkpoint-related proteins as predictors of tumor recurrence and survival in CCRCC and sustain sTIM3 and sBTLA, but did not predict worse survival for sPD-L1 [
41].
According to our experience, both PD-1 and PD-L1 immunohistochemical expression are associated with well-recognized histopathologic parameters of tumor aggressiveness and PD-L1 is also an independent marker of prognosis in our series, both on the tumor center and invasive fronts. Notably, this is a population of patients with CCRCC including all stages and not necessarily treated with antiangiogenic therapy or immune checkpoint inhibition therapy. What is more, we have simultaneously evaluated PD-1 and PD-L1 both in the tumor and serum of the same cohort of patients and have confirmed that sPD-L1 is definitely an independent prognostic factor that is non-associated with the tumor size, Fuhrman grade or histopathological staging. Multivariate analysis revealed that sPD-L1 > 793 ng/mL is associated with worse survival (HR 8.67), together with pT category (HR 2.24) or presence of metastasis (HR 2.83). We also confirmed a major variation in sPD-L1 levels according to the time of the metastatic event, with a much higher expression in synchronous metastases than in metachronous ones. No less interesting is the fact that a positive PD-L1 expression in the tumor center and the invading tumor front— as well as as PD-L1 level > 793 ng/mL—leads to a worse overall survival rate in CCRCC patients. However, what is even more interesting in our experience is that sPDL-1 levels appear to represent a good surrogate of a response criteria to systemic therapy administered in metastatic CCRCC. In this context, there are many limitations to consider when deciding to treat CCRCC patients with immune checkpoint inhibitors based on the immunohistochemical detection of PD-1/PD-L1 positivity alone [
11,
27], while the search of markers to anticipate the response to immunotherapy continues [
42,
43,
44]. A new trial (UMIN000027873) has been recently launched to evaluate the therapeutic effect of nivolumamb as a second-line therapy for advanced CCRCC based on the concentrations of serum sPD-L1. The hypothesis of this study is that patients with high blood levels of sPD-L1 will experience a greater therapeutic effect during nivolumab treatment [
45].
The limitations of our study include its retrospective nature, despite the fact that the cases were prospectively followed after tumor and serum samples were obtained. Patients were treated using state-of-the art procedures, and size of the sample was also relatively small, especially when different subsets of patients were specifically analyzed. Also, our finding that sPD-1 levels in controls are higher than in CCRCC patients could be explained by a confounding effect of disparity levels in CCRCC patients due to the fact that the sample includes patients with all stages of disease. It also could have been due to inappropriateness of the control sample as a result of unknown factors. Regardless, we did not test the hypothesis that sPD-L1 can be a tumor marker for the diagnosis of CCRCC, but we did show evidence supporting the idea that it can be a good marker to evaluate prognosis for CCRCC patients when they are taken as a whole, and also in the subset of metastatic patients being treated with the IMDC model. Also, we support its use as a marker of prognosis in metastatic patients treated with systemic therapies, mainly TKIs.
Future studies should try to evaluate the role of sPD-L1 and other soluble immune checkpoint-related proteins to elucidate their role as intrinsic tumor markers with utility in prognostic evaluation involving CCRCC as a malignancy without markers of clinical value, despite the great therapeutic success that has been achieved in the last decade.
4. Materials and Methods
The present study including all of its experiments comply with current Spanish and European Union legal regulations. The Basque Biobank for Research-OEHUN (
www.biobancovasco.org) was employed the source of samples and data from patients that could be used for research purposes. Each patient signed a specific document which had been approved by the Ethical and Scientific Committees of the Basque Country Public Health System (Osakidetza) (PI + CES-BIOEF 2018-04).
4.1. Patients
Plasma samples and tumor tissues were obtained from 89 CCRCC patients that were surgically treated at Basurto University Hospital from 2012 to 2016. The plasma samples were preoperatively collected for the study. Patients with non-metastatic CCRCC were treated surgically and patients with metastatic disease received nephrectomy and systemic therapy according to their ICDM classification, age and clinical condition.
Sixty patients were males (mean age: 60.83 years; range: 36–82) and 29 were females (mean age: 62.69; range: 32–80). Pathological characteristics are summarized in
Table 3. Plasma from 46 healthy volunteers with no clinical history of neoplastic diseases was used as control samples (male/female 28/18, age 55.8/61.8 years).
Samples from the center (
n = 88) and the infiltration front (
n = 75) of tumors from these patients were distinguished in the histopathological department and included in tissue microarrays (TMAs) for further immunohistochemical analyses. American Joint Committee on Cancer (AJCC) [
19] and Furhman’s [
20] methods were applied to assign the relevant stage and grade, respectively.
During the follow-up (mean: 59.9 months, range: 1–91 months), 21 patients were found to no longer be alive and 68 were still alive. All patients were prospectively followed until death or the last-follow-up. The cause and date of death was taken as specified in clinical records and overall survival (OS) was investigated.
4.2. IMDC Model and MASS Response Criteria for Patients with Metastatic CCRCC
The International mRCC Database Consortium (IMDC) represents the largest collection of real-world data on patients with advanced kidney cancer treated with targeted therapies. The IMDC prognostic model has been used to stratify patients in contemporary clinical trials and to provide risk-directed treatment selection in everyday clinical practice. This model classifies metastatic patients into three categories at different risk of death: favorable, intermediate and poor risk [
46]. We used the IMDC to evaluate the group of patients with metastatic CCRCC in this series (
n = 23).
In order to evaluate the response assessment to systemic therapy in metastatic CCRCC receiving treatments other than nephrectomy (
n = 16), the Morphology, Attenuation, Size and Structure (MASS) criteria was used to distinguish between the three categories of patients. Patients with a favorable response to therapy are those with no new lesions displayed on imaging modalities and any of the following outcomes: i. A decrease in the tumor size of ≥20%; ii. One or more predominantly solid enhancing lesions showed marked central necrosis or marked decreased attenuation (≥40 Hounsfield units). Patients with an unfavorable response are those with either: i. An increase in the tumor size of ≥20% in the absence of marked central necrosis or marked decreased attenuation; ii. New metastases, marked central fill-in or new enhancement of a previously homogeneously hypoattenuating non-enhancing mass. Patients with an indeterminate response are those who do not fit the criteria for favorable or unfavorable responses [
47].
4.3. Immunohistochemistry
PD-L1 and PD-1 was analyzed in formalin-fixed and paraffin-embedded material using specific antibodies (PD-1 (Ventana, clone NAT105, ready-to-use) and PD-L1 (Ventana, clone SP-142, ready-to-use)). Immunostaining was performed using an automated immunostainer (Benchmark Ultra, Ventana, Roche, AZ, USA) following the protocols recommended by the manufacturer.
We documented the presence (+) or absence (−) of PD-L1 and PD-1 immunolabels in inflammatory cells [
27] using a Nikon Eclipse 80i microscope (Nikon, Tokyo, Japan). All specimens were independently evaluated by two observers; in the event of discrepancies, samples were re-evaluated to arrive at a final conclusion.
4.4. ELISA Assays
Levels of soluble PD-L1 and PD-1 were evaluated using the human B7-H1 and PD-1 DuoSet ELISA kits (R&D Systems, DY156 and DY1086, respectively) according to the manufacturer’s protocols [
37]. Briefly, 96 well plates were coated with capture antibodies diluted in PBS and incubated overnight at 4 °C. After washing, plates were blocked in order to avoid unspecific binding. Standards (100 µL) together with optimized plasma sample dilutions (1/8 for sPD-L1 and 1/4 for sPD-1) and controls were added to the wells and incubated for 2 h at room temperature (RT). After washing the plate, 100 µL/well of biotinylated detection antibody was added and incubated for 1 h at RT. Subsequently, Streptavidin-HRP A solution was added and the mixture was incubated for 20 min. Finally, following multiple washes, the wells were incubated with 100 µL/well of Substrate Solution and were stopped after 20 min with 2N H2SO4. The readout was made by reading the absorbance at 450 nm with a FluoStar Optima plate reader (BMG Labtech). The amount of protein of interest in the sample was estimated using a standard curve after applying the dilution factor.
4.5. Statistical Analysis
The statistical analysis was performed by using SPSS® 24.0 software. In order to assess whether data obtained from the tissue and plasma samples followed a normal distribution, we applied a Kolmogorov-Smirnov test. Based on this information, data were further analyzed using parametric or non-parametric tests.
The Spearman Rho test was used to test the correlation between tumor tissue PD-L1 and PD-1 expression, sPD-L1 and sPD-1 levels and patient age and gender. Comparison of plasma levels of sPD-L1 and sPD-1 between two groups or more (respectively) was carried out using the Mann-Whitney (Mann-U) and Kruskal-Wallis tests. To analyze categorical tissue expression of PD-L1 and PD-1 (negative/positive) and to test the association of differences with pathological variables, we used the Chi-square (χ2) test.
Overall survival (OS) analyses were performed following the establishing of groups by cut-off points, following different methods: (I) for tissue analyses, cut-off points were based on the categorical expression of PD-L1 and PD-1 (negative (<1% staining) vs. positive (≥1% staining)); (II) a classification and regression tree (CRT) method was employed for the analysis of plasma sPD-L1 and sPD-1; (III) in order to evaluate the OS of CCRCC patients, Kaplan-Meier curves and log-rank tests were utilized; (IV) to evaluate the independent effects of PD-L1 and PD-1 expression and plasma levels of soluble isoforms and pathological variables on OS, we employed multivariate analyses (the Cox regression model with the backward Wald stepwise method).