Loss of microRNA-135b Enhances Bone Metastasis in Prostate Cancer and Predicts Aggressiveness in Human Prostate Samples
Abstract
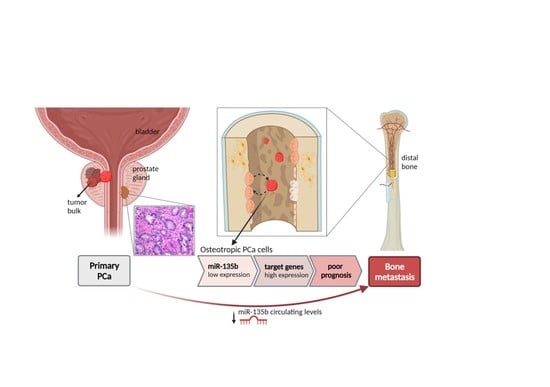
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Animal Model
2.3. Study Subjects
2.3.1. Laser Capture Microdissection (LCM)
2.3.2. Plasma Collection
2.4. In Vitro Assays
2.4.1. Transient Transfection of miRNA Mimics
2.4.2. Proliferation Analysis
2.4.3. Transwell Migration Assay
2.4.4. 3′-UTR Cloning and Luciferase Assay
2.5. Molecular Characterization of PC3 Bone-Metastatic Subclones
2.5.1. RNA and miRNA Isolation
2.5.2. MicroRNA Profiling Analysis
2.5.3. Transcriptomic Array
2.5.4. Gene and miRNA Expression
2.5.5. miRNA Expression in Plasma
2.6. Bioinformatics Analysis
2.6.1. Pathway Analysis
2.6.2. Analysis of miRNA Targets
2.6.3. Analysis of PCa Genomic and Transcriptomic Datasets
3. Results
3.1. In Vivo Selection for Bone-Metastasizing PC3 Cells
3.2. Transcriptional Analysis of PC3-BM Cells Revealed Multiple Bone Metastatic-Related Genes
3.3. Differential miRNA Expression Profile of In Vivo Selected Metastatic Prostate Cancer Cells
3.4. Overexpression of miR-135b, miR-200b, and miR-19a Decreases Migration of PC3-BM Cells
3.5. Integration Data Analysis Identifies Biomarkers of Metastasis and Pathways Related to PCa Bone Metastases
3.6. miR-135b Regulates JAKMIP2, PLAG1, PDGFA, and VTI1 Levels
3.7. miR-135b and Its Targets, JAKMIP2, PLAG1, and PDGFA, Correlate with Poor Prognosis of PCa Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.B.F. Global Cancer Observatory: Cancer Today; International Agency for Research on Cancer: Lyon, France, 2020. Available online: https://gco.iarc.fr/today (accessed on 30 April 2021).
- Tsuzuki, S.; Park, S.H.; Eber, M.R.; Peters, C.; Shiozawa, Y. Skeletal complications in cancer patients with bone metastases. Int. J. Urol. 2016, 23, 825–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, S.S.; Stockert, J.A.; Hackert, V.; Yadav, K.K.; Tewari, A.K. Intratumor Heterogeneity in Prostate Cancer. Urol. Oncol. Semin. Orig. Investig. 2018, 36, 349–360. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Cano, S.J. Tumor Heterogeneity: Mechanisms and Bases for a Reliable Application of Molecular Marker Design. Int. J. Mol. Sci. 2012, 13, 1951–2011. [Google Scholar] [CrossRef] [PubMed]
- Weilbaecher, K.N.; Guise, T.A.; McCauley, L.K. Cancer to bone: A fatal attraction. Nat. Rev. Cancer 2011, 11, 411–425. [Google Scholar] [CrossRef]
- Singh, A.S.; Figg, W.D. In vivo models of prostate cancer metastasis to bone. J. Urol. 2005, 174, 820–826. [Google Scholar] [CrossRef]
- Raheem, O.; Kulidjian, A.A.; Wu, C.; Jeong, Y.B.; Yamaguchi, T.; Smith, K.M.; Goff, D.; Leu, H.; Morris, S.R.; Cacalano, N.A.; et al. A novel patient-derived intra-femoral xenograft model of bone metastatic prostate cancer that recapitulates mixed osteolytic and osteoblastic lesions. J. Transl. Med. 2011, 9, 185. [Google Scholar] [CrossRef] [Green Version]
- Croset, M.; Kan, C.; Clézardin, P. Tumour-derived miRNAs and bone metastasis. BoneKEy Rep. 2015, 4, 688. [Google Scholar] [CrossRef] [Green Version]
- Kanwal, R.; Plaga, A.R.; Liu, X.; Shukla, G.C.; Gupta, S. MicroRNAs in prostate cancer: Functional role as biomarkers. Cancer Lett. 2017, 407, 9–20. [Google Scholar] [CrossRef]
- Schaefer, A.; Stephan, C.; Busch, J.; Yousef, G.M.; Jung, K. Diagnostic, prognostic and therapeutic implications of microRNAs in urologic tumors. Nat. Rev. Urol. 2010, 7, 286–297. [Google Scholar] [CrossRef]
- Sharma, N.; Baruah, M.M. The microRNA signatures: Aberrantly expressed miRNAs in prostate cancer. Clin. Transl. Oncol. 2018, 21, 126–144. [Google Scholar] [CrossRef]
- Khanmi, K.; Ignacimuthu, S.; Paulraj, M.G. MicroRNA in Prostate Cancer. Clin. Chim. Acta 2015, 451, 154–160. [Google Scholar] [CrossRef]
- Goto, Y.; Kojima, S.; Nishikawa, R.; Kurozumi, A.; Kato, M.; Enokida, H.; Matsushita, R.; Yamazaki, K.; Ishida, Y.; Nakagawa, M.; et al. MicroRNA expression signature of castration-resistant prostate cancer: The microRNA-221/222 cluster functions as a tumour suppressor and disease progression marker. Br. J. Cancer 2015, 113, 1055–1065. [Google Scholar] [CrossRef] [PubMed]
- Kato, M.; Goto, Y.; Matsushita, R.; Kurozumi, A.; Fukumoto, I.; Nishikawa, R.; Kamoto, S.; Enokida, H.; Nakagawa, M.; Ichikawa, T.; et al. MicroRNA-26a/b directly regulate La-related protein 1 and inhibit cancer cell invasion in prostate cancer. Int. J. Oncol. 2015, 47, 710–718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurozumi, A.; Goto, Y.; Matsushita, R.; Fukumoto, I.; Kato, M.; Nishikawa, R.; Sakamoto, S.; Enokida, H.; Nakagawa, M.; Ichikawa, T.; et al. Tumor-suppressive micro RNA-223 inhibits cancer cell migration and invasion by targeting ITGA 3/ ITGB 1 signaling in prostate cancer. Cancer Sci. 2015, 107, 84–94. [Google Scholar] [CrossRef] [Green Version]
- Bonci, D.; Coppola, V.; Patrizii, M.; Addario, A.; Cannistraci, A.; Francescangeli, F.; Pecci, R.; Muto, G.; Collura, D.; Bedini, R.; et al. A microRNA code for prostate cancer metastasis. Oncogene 2015, 35, 1180–1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Z.; Wen, Y.; Xuan, C.; Chen, Q.; Xiang, Q.; Wang, J.; Liu, Y.; Luo, L.; Zhao, S.; Deng, Y.; et al. Identifying the key genes and microRNAs in prostate cancer bone metastasis by bioinformatics analysis. FEBS Open Bio 2020, 10, 674–688. [Google Scholar] [CrossRef]
- Dai, Y.; Wu, Z.; Lang, C.; Zhang, X.; He, S.; Yang, Q.; Guo, W.; Lai, Y.; Du, H.; Peng, X.; et al. Copy number gain of ZEB1 mediates a double-negative feedback loop with miR-33a-5p that regulates EMT and bone metastasis of prostate cancer dependent on TGF-β signaling. Theranostics 2019, 9, 6063–6079. [Google Scholar] [CrossRef]
- Fang, L.-L.; Sun, B.-F.; Huang, L.-R.; Yuan, H.-B.; Zhang, S.; Chen, J.; Yu, Z.-J.; Luo, H. Potent Inhibition of miR-34b on Migration and Invasion in Metastatic Prostate Cancer Cells by Regulating the TGF-β Pathway. Int. J. Mol. Sci. 2017, 18, 2762. [Google Scholar] [CrossRef] [Green Version]
- Ren, N.; Wang, M.; Guo, W.; Zhao, X.; Tu, X.; Huang, S.; Zou, X.; Peng, X. Wild-type p53 suppresses the epithelial-mesenchymal transition and stemness in PC-3 prostate cancer cells by modulating miR-145. Int. J. Oncol. 2013, 42, 1473–1481. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Ji, Z.; Yan, W.; Zhou, Z.; Li, H. The biological functions and mechanism of miR-212 in prostate cancer proliferation, migration and invasion via targeting Engrailed-2. Oncol. Rep. 2017, 38, 1411–1419. [Google Scholar] [CrossRef] [Green Version]
- Kong, D.; Li, Y.; Wang, Z.; Banerjee, S.; Ahmad, A.; Kim, H.C.; Sarkar, F.H. miR-200 Regulates PDGF-D-Mediated Epithelial–Mesenchymal Transition, Adhesion, and Invasion of Prostate Cancer Cells. Stem Cells 2009, 27, 1712–1721. [Google Scholar] [CrossRef] [Green Version]
- Seashols-Williams, S.J.; Budd, W.; Clark, G.C.; Wu, Q.; Daniel, R.; Dragoescu, E.; Zehner, Z.E. miR-9 Acts as an OncomiR in Prostate Cancer through Multiple Pathways That Drive Tumour Progression and Metastasis. PLoS ONE 2016, 11, e0159601. [Google Scholar] [CrossRef] [Green Version]
- MiR-181a Promotes Epithelial to Mesenchymal Transition of Prostate Cancer Cells by Targeting TGIF2. Available online: https://pubmed.ncbi.nlm.nih.gov/29164579/ (accessed on 18 November 2021).
- Ren, D.; Yang, Q.; Dai, Y.; Guo, W.; Du, H.; Song, L.; Peng, X. Oncogenic miR-210-3p promotes prostate cancer cell EMT and bone metastasis via NF-κB signaling pathway. Mol. Cancer 2017, 16, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Q.; Gao, Y.; Yang, F.; Mao, T.; Sun, Z.; Wang, H.; Song, B.; Li, X. Suppression of microRNA-454 impedes the proliferation and invasion of prostate cancer cells by promoting N-myc downstream-regulated gene 2 and inhibiting WNT/β-catenin signaling. Biomed. Pharmacother. 2018, 97, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Oh-Hohenhorst, S.J.; Lange, T. Role of Metastasis-Related microRNAs in Prostate Cancer Progression and Treatment. Cancers 2021, 13, 4492. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-W.; Chang, Y.-L.; Chang, Y.-C.; Lin, J.-C.; Chen, C.-C.; Pan, S.-H.; Wu, C.-T.; Chen, H.-Y.; Yang, S.-C.; Hong, T.-M.; et al. MicroRNA-135b promotes lung cancer metastasis by regulating multiple targets in the Hippo pathway and LZTS1. Nat. Commun. 2013, 4, 1877. [Google Scholar] [CrossRef] [Green Version]
- Huangfu, L.; He, Q.; Han, J.; Shi, J.; Li, X.; Cheng, X.; Guo, T.; Du, H.; Zhang, W.; Gao, X.; et al. MicroRNA-135b/CAMK2D Axis Contribute to Malignant Progression of Gastric Cancer through EMT Process Remodeling. Int. J. Biol. Sci. 2021, 17, 1940–1952. [Google Scholar] [CrossRef]
- Valeri, N.; Braconi, C.; Gasparini, P.; Murgia, C.; Lampis, A.; Paulus-Hock, V.; Hart, J.R.; Ueno, L.; Grivennikov, S.I.; Lovat, F.; et al. MicroRNA-135b Promotes Cancer Progression by Acting as a Downstream Effector of Oncogenic Pathways in Colon Cancer. Cancer Cell 2014, 25, 469–483. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Wang, H.; Che, J.; Xu, L.; Yang, W.; Li, Y.; Zhou, W. Silencing of microRNA-135b inhibits invasion, migration, and stemness of CD24+CD44+ pancreatic cancer stem cells through JADE-1-dependent AKT/mTOR pathway. Cancer Cell Int. 2020, 20, 134. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, F.; Zhang, F.; Cui, Y.; Wang, Q.; Liu, H.; Wu, Y. miR-135b-5p enhances doxorubicin-sensitivity of breast cancer cells through targeting anterior gradient 2. J. Exp. Clin. Cancer Res. 2019, 38, 26. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Zhang, G.; Li, J.; Liu, J.; Lv, P. The Tumor-Suppressive MicroRNA-135b Targets c-Myc in Osteoscarcoma. PLoS ONE 2014, 9, e102621. [Google Scholar] [CrossRef]
- Aakula, A.; Leivonen, S.-K.; Hintsanen, P.; Aittokallio, T.; Ceder, Y.; Børresen-Dale, A.-L.; Perälä, M.; Östling, P.; Kallioniemi, O. MicroRNA-135b regulates ERα, AR and HIF1AN and affects breast and prostate cancer cell growth. Mol. Oncol. 2015, 9, 1287–1300. [Google Scholar] [CrossRef] [PubMed]
- Chunjiao, S.; Huan, C.; Chaoyang, X.; Guomei, R. Uncovering the roles of miRNAs and their relationship with androgen receptor in prostate cancer. IUBMB Life 2014, 66, 379–386. [Google Scholar] [CrossRef] [Green Version]
- Östling, P.; Leivonen, S.-K.; Aakula, A.; Kohonen, P.; Mäkelä, R.; Hagman, Z.; Edsjö, A.; Kangaspeska, S.; Edgren, H.; Nicorici, D.; et al. Systematic Analysis of MicroRNAs Targeting the Androgen Receptor in Prostate Cancer Cells. Cancer Res. 2011, 71, 1956–1967. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Tao, L.; Zhong, H.; Zhao, S.; Yu, Y.; Yu, B.; Chen, X.; Gao, J.; Wang, R. miR-135b inhibits tumour metastasis in prostate cancer by targeting STAT6. Oncol. Lett. 2015, 11, 543–550. [Google Scholar] [CrossRef]
- Zoni, E.; van der Pluijm, G. The role of microRNAs in bone metastasis. J. Bone Oncol. 2016, 5, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Gordanpour, A.; Nam, R.K.; Sugar, L.; Seth, A. MicroRNAs in prostate cancer: From biomarkers to molecularly-based therapeutics. Prostate Cancer Prostatic Dis. 2012, 15, 314–319. [Google Scholar] [CrossRef]
- El Sayed, S.R.; Cristante, J.; Guyon, L.; Denis, J.; Chabre, O.; Cherradi, N. MicroRNA Therapeutics in Cancer: Current Advances and Challenges. Cancers 2021, 13, 2680. [Google Scholar] [CrossRef]
- Garcia, M.; Vélez, R.; Romagosa, C.; Majem, B.; Pedrola, N.; Oliván, M.; Rigau, M.; Guiu, M.; Gomis, R.R.; Morote, J.; et al. Cyclooxygenase-2 inhibitor suppresses tumour progression of prostate cancer bone metastases in nude mice. BJU Int. 2014, 113, E164–E177. [Google Scholar] [CrossRef] [Green Version]
- Harms, J.F.; Welch, D.R. MDA-MB-435 human breast carcinoma metastasis to bone. Clin. Exp. Metastasis 2003, 20, 327–334. [Google Scholar] [CrossRef]
- Bateman, A.; Martin, M.J.; O’Donovan, C.; Magrane, M.; Apweiler, R.; Alpi, E.; Antunes, R.; Arganiska, J.; Bely, B.; Bingley, M.; et al. UniProt: A Hub for Protein Information. Nucleic Acids Res. 2015, 43, D204–D212. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S.; Sato, Y.; Kawashima, M.; Furumichi, M.; Tanabe, M. Data, information, knowledge and principle: Back to metabolism in KEGG. Nucleic Acids Res. 2013, 42, D199–D205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valls, R.; Pujol, A.; Farrés, J.; Artigas, L.; Mas, J.M. Anaxomics’ Methodologies-Understanding the Complexity of Biological Processes; Anaxomics: Barcelona, Spain, 2012. [Google Scholar]
- Whirl-Carrillo, M.; McDonagh, E.M.; Hebert, J.M.; Gong, L.; Sangkuhl, K.; Thorn, C.F.; Altman, R.B.; Klein, T.E. Pharmacogenomics Knowledge for Personalized Medicine. Clin. Pharmacol. Ther. 2012, 92, 414–417. [Google Scholar] [CrossRef] [PubMed]
- Frolkis, A.; Knox, C.; Lim, E.; Jewison, T.; Law, V.; Hau, D.D.; Liu, P.; Gautam, B.; Ly, S.; Guo, A.C.; et al. SMPDB: The Small Molecule Pathway Database. Nucleic Acids Res. 2009, 38, D480–D487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, H.; Cho, J.-W.; Lee, S.-Y.; Yun, A.; Kim, H.; Bae, D.; Yang, S.; Kim, C.Y.; Lee, M.; Kim, E.; et al. TRRUST v2: An expanded reference database of human and mouse transcriptional regulatory interactions. Nucleic Acids Res. 2017, 46, D380–D386. [Google Scholar] [CrossRef]
- Agarwal, V.; Bell, G.W.; Nam, J.-W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. eLife 2015, 4, e05005. [Google Scholar] [CrossRef]
- Betel, D.; Koppal, A.; Agius, P.; Sander, C.; Leslie, C. Comprehensive modeling of microRNA targets predicts functional non-conserved and non-canonical sites. Genome Biol. 2010, 11, R90. [Google Scholar] [CrossRef] [Green Version]
- Dweep, H.; Gretz, N. MiRWalk2.0: A Comprehensive Atlas of MicroRNA-Target Interactions. Nat. Methods 2015, 12, 697. [Google Scholar] [CrossRef]
- Krüger, J.; Rehmsmeier, M. RNAhybrid: microRNA target prediction easy, fast and flexible. Nucleic Acids Res. 2006, 34 (Suppl. 2), W451–W454. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative Analysis of Complex Cancer Genomics and Clinical Profiles Using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.; Wang, Z.; Yang, P.; Yang, J.; Liang, J.; Chen, Y.; Wang, H.; Wei, G.; Ye, S.; Zhou, Y. MicroRNA-135b regulates metastasis suppressor 1 expression and promotes migration and invasion in colorectal cancer. Mol. Cell. Biochem. 2013, 388, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef] [PubMed]
- Gdowski, A.S.; Ranjan, A.; Vishwanatha, J. Current concepts in bone metastasis, contemporary therapeutic strategies and ongoing clinical trials. J. Exp. Clin. Cancer Res. 2017, 36, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Distribution of Secondary Growths in Cancer of the Breast. 1889. Available online: https://pubmed.ncbi.nlm.nih.gov/2673568/ (accessed on 3 May 2021).
- Nunez-Olle, M.; Guiu, M.; Gomis, R.R. In Vivo Assessment of Metastatic Cell Potential in Prostate Cancer. Methods Mol. Biol. 2021, 2294, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-X.; Wang, J.; Shelburne, C.E.; Lopatin, D.E.; Chinnaiyan, A.M.; Rubin, M.; Pienta, K.; Taichman, R.S. Expression of CXCR4 and CXCL12 (SDF-1) in human prostate cancers (PCa) in vivo. J. Cell. Biochem. 2003, 89, 462–473. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Loberg, R.; Liao, J.; Ying, C.; Snyder, L.A.; Pienta, K.; McCauley, L.K. A Destructive Cascade Mediated by CCL2 Facilitates Prostate Cancer Growth in Bone. Cancer Res. 2009, 69, 1685–1692. [Google Scholar] [CrossRef] [Green Version]
- Han, H.H.; Gil Kim, B.; Lee, J.H.; Kang, S.; Kim, J.E.; Cho, N.H. Angiopoietin-2 promotes ER+ breast cancer cell survival in bone marrow niche. Endocrine-Relat. Cancer 2016, 23, 609–623. [Google Scholar] [CrossRef] [Green Version]
- D’Oronzo, S.; Brown, J.; Coleman, R. The role of biomarkers in the management of bone-homing malignancies. J. Bone Oncol. 2017, 9, 1–9. [Google Scholar] [CrossRef]
- Panigrahi, G.; Ramteke, A.; Birks, D.; Ali, H.E.A.; Venkataraman, S.; Agarwal, C.; Vibhakar, R.; Miller, L.; Agarwal, R.; Elmageed, Z.Y.A.; et al. Exosomal microRNA profiling to identify hypoxia-related biomarkers in prostate cancer. Oncotarget 2018, 9, 13894–13910. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Zou, C.; Tang, Y.; Wa, Q.; Peng, X.; Chen, X.; Yang, C.; Ren, D.; Huang, Y.; Liao, Z.; et al. miR-582-3p and miR-582-5p Suppress Prostate Cancer Metastasis to Bone by Repressing TGF-β Signaling. Mol. Ther.-Nucleic Acids 2019, 16, 91–104. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.-Y.; Liu, S.-Y.; Chang, Y.-S.; Yin, J.J.; Yeh, H.-L.; Mouhieddine, T.; Hadadeh, O.; Abou-Kheir, W.; Liu, Y.-N. MicroRNA-34a regulates WNT/TCF7 signaling and inhibits bone metastasis in Ras-activated prostate cancer. Oncotarget 2014, 6, 441–457. [Google Scholar] [CrossRef] [Green Version]
- Gaur, S.; Wen, Y.; Song, J.H.; Parikh, N.U.; Mangala, L.S.; Blessing, A.M.; Ivan, C.; Wu, S.Y.; Varkaris, A.; Shi, Y.; et al. Chitosan nanoparticle-mediated delivery of miRNA-34a decreases prostate tumor growth in the bone and its expression induces non-canonical autophagy. Oncotarget 2015, 6, 29161–29177. [Google Scholar] [CrossRef] [Green Version]
- Xia, L.; Han, Q.; Chi, C.; Zhu, Y.; Pan, J.; Dong, B.; Huang, Y.; Xia, W.; Xue, W.; Sha, J. Transcriptional regulation of PRKAR2B by miR-200b-3p/200c-3p and XBP1 in human prostate cancer. Biomed. Pharmacother. 2020, 124, 109863. [Google Scholar] [CrossRef]
- He, M.; Liu, Y.; Deng, X.; Qi, S.; Sun, X.; Liu, G.; Liu, Y.; Liu, Y.; Zhao, M. Down-regulation of miR-200b-3p by low p73 contributes to the androgen-independence of prostate cancer cells. Prostate 2013, 73, 1048–1056. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, H.; Liu, Y.; Su, P.; Zhang, J.; Wang, X.; Sun, M.; Chen, B.; Zhao, W.; Wang, L.; et al. SREBP1, targeted by miR-18a-5p, modulates epithelial-mesenchymal transition in breast cancer via forming a co-repressor complex with Snail and HDAC1/2. Cell Death Differ. 2018, 26, 843–859. [Google Scholar] [CrossRef] [PubMed]
- Kolenda, T.; Guglas, K.; Kopczyńska, M.; Sobocińska, J.; Teresiak, A.; Bliźniak, R.; Lamperska, K. Good or Not Good: Role of MiR-18a in Cancer Biology. Rep. Pract. Oncol. Radiother. 2020, 25, 808–819. [Google Scholar] [CrossRef]
- MiR-210-3p Inhibits the Tumor Growth and Metastasis of Bladder Cancer via Targeting Fibroblast Growth Factor Receptor-like 1. Available online: https://pubmed.ncbi.nlm.nih.gov/28861329/ (accessed on 30 April 2021).
- Hong, Y.-G.; Huang, Z.-P.; Liu, Q.-Z.; E, J.-F.; Gao, X.-H.; Xin, C.; Zhang, W.; Li, P.; Hao, L.-Q. MicroRNA-95-3p inhibits cell proliferation and metastasis in colorectal carcinoma by HDGF. Biomed. J. 2020, 43, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.J.; Lee, H.W.; Kim, H.R.; Song, H.J.; Lee, D.H.; Lee, H.; Shin, C.H.; Joung, J.-G.; Kim, D.-H.; Joo, K.M.; et al. Overexpression of microRNA-95-3p suppresses brain metastasis of lung adenocarcinoma through downregulation of cyclin D1. Oncotarget 2015, 6, 20434–20448. [Google Scholar] [CrossRef] [Green Version]
- MiR-425-5p Suppresses Tumorigenesis and DDP Resistance in Human-Prostate Cancer by Targeting GSK3β and Inactivating the Wnt/β-Catenin Signaling Pathway. Available online: https://pubmed.ncbi.nlm.nih.gov/31502580/ (accessed on 30 April 2021).
- Zhang, J.-Y.; Su, X.-P.; Li, Y.-N.; Guo, Y.-H. MicroRNA-425-5p promotes the development of prostate cancer via targeting forkhead box J3. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 547–554. [Google Scholar] [PubMed]
- Wu, D.-M.; Wen, X.; Han, X.-R.; Wang, S.; Wang, Y.-J.; Shen, M.; Fan, S.-H.; Zhang, Z.-F.; Shan, Q.; Li, M.-Q.; et al. Bone Marrow Mesenchymal Stem Cell-Derived Exosomal MicroRNA-126-3p Inhibits Pancreatic Cancer Development by Targeting ADAM9. Mol. Ther.-Nucleic Acids 2019, 16, 229–245. [Google Scholar] [CrossRef] [Green Version]
- Shen, P.-F.; Chen, X.-Q.; Liao, Y.-C.; Chen, N.; Zhou, Q.; Wei, Q.; Li, X.; Wang, J.; Zeng, H. MicroRNA-494-3p targets CXCR4 to suppress the proliferation, invasion, and migration of prostate cancer. Prostate 2014, 74, 756–767. [Google Scholar] [CrossRef]
- Lee, K.-H.; Lin, F.-C.; Hsu, T.-I.; Lin, J.-T.; Guo, J.-H.; Tsai, C.-H.; Lee, Y.-C.; Chen, C.-L.; Hsiao, M.; Lu, P.-J. MicroRNA-296-5p (miR-296-5p) functions as a tumor suppressor in prostate cancer by directly targeting Pin1. Biochim. Biophys. Acta (BBA)-Bioenerg. 2014, 1843, 2055–2066. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Chen, Q.; Yan, J.; Wang, Y.; Zhu, C.; Chen, C.; Zhao, X.; Xu, M.; Sun, Q.; Deng, R.; et al. MiRNA-296-3p-ICAM-1 axis promotes metastasis of prostate cancer by possible enhancing survival of natural killer cell-resistant circulating tumour cells. Cell Death Dis. 2013, 4, e928. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, J.; Qiu, J.; Fu, X.; Tang, Q.; Yang, F.; Zhao, Z.; Wang, H. MicroRNA-382 inhibits prostate cancer cell proliferation and metastasis through targeting COUP-TFII. Oncol. Rep. 2016, 36, 3707–3715. [Google Scholar] [CrossRef]
- Das, R.; Gregory, P.; Fernandes, R.C.; Denis, I.; Wang, Q.; Townley, S.L.; Zhao, S.G.; Hanson, A.R.; Pickering, M.A.; Armstrong, H.; et al. MicroRNA-194 Promotes Prostate Cancer Metastasis by Inhibiting SOCS2. Cancer Res. 2016, 77, 1021–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, Q.; Chen, X.-S.; Tian, T.; Xia, X.-Y.; Xu, P. MicroRNA-194 suppresses prostate cancer migration and invasion by downregulating human nuclear distribution protein. Oncol. Rep. 2016, 37, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Wa, Q.; Li, L.; Lin, H.; Peng, X.; Ren, N.; Huang, Y.; He, P.; Huang, S. Downregulation of miR-19a-3p promotes invasion, migration and bone metastasis via activating TGF-β signaling in prostate cancer. Oncol. Rep. 2018, 39, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Lv, Z.; Xin, H.; Yang, Z.; Wang, W.; Dong, J.; Jin, L.; Li, F. miR-135b promotes proliferation and metastasis by targeting APC in triple-negative breast cancer. J. Cell. Physiol. 2018, 234, 10819–10826. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.H.; Xin, Z.M. MiR-135b-5p Inhibits the Progression of Malignant Melanoma Cells by Tar-geting RBX1. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 1309–1315. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, P.; Suva, L.J.; Welch, D.R.; Donahue, H.J. Osteoprotegrin and the bone homing and colonization potential of breast cancer cells. J. Cell. Biochem. 2007, 103, 30–41. [Google Scholar] [CrossRef]
- Carlini, M.J.; De Lorenzo, M.S.; Puricelli, L. Cross-talk between tumor cells and the microenvironment at the metastatic niche. Curr. Pharm. Biotechnol. 2011, 12, 1900–1908. [Google Scholar] [CrossRef] [PubMed]
- Steinbichler, T.B.; Dudas, J.; Riechelmann, H.; Skvortsova, I.-I. The role of exosomes in cancer metastasis. Semin. Cancer Biol. 2017, 44, 170–181. [Google Scholar] [CrossRef]
- Becker, A.; Thakur, B.K.; Weiss, J.M.; Kim, H.S.; Peinado, H.; Lyden, D. Extracellular Vesicles in Cancer: Cell-to-Cell Mediators of Metastasis. Cancer Cell 2016, 30, 836–848. [Google Scholar] [CrossRef] [Green Version]
- Sahraei, M.; Roy, L.D.; Curry, J.M.; Teresa, T.L.; Nath, S.; Besmer, D.; Kidiyoor, A.; Dalia, R.; Gendler, S.J.; Mukherjee, P. MUC1 regulates PDGFA expression during pancreatic cancer progression. Oncogene 2012, 31, 4935–4945. [Google Scholar] [CrossRef] [Green Version]
- Castaneda, C.A.; Cortes-Funes, H.; Gomez, H.L.; Ciruelos, E.M. The phosphatidyl inositol 3-kinase/AKT signaling pathway in breast cancer. Cancer Metastasis Rev. 2010, 29, 751–759. [Google Scholar] [CrossRef]
- Li, L.; Ameri, A.H.; Wang, S.; Jansson, K.H.; Casey, O.M.; Yang, Q.; Beshiri, M.; Fang, L.; Lake, R.G.; Agarwal, S.; et al. EGR1 regulates angiogenic and osteoclastogenic factors in prostate cancer and promotes metastasis. Oncogene 2019, 38, 6241–6255. [Google Scholar] [CrossRef] [PubMed]
- Thulin, M.H.; Jennbacken, K.; Damber, J.-E.; Welén, K. Osteoblasts stimulate the osteogenic and metastatic progression of castration-resistant prostate cancer in a novel model for in vitro and in vivo studies. Clin. Exp. Metastasis 2013, 31, 269–283. [Google Scholar] [CrossRef] [Green Version]
- Jin, L.; Chun, J.; Pan, C.; Kumar, A.; Zhang, G.; Ha, Y.; Li, D.; Alesi, G.N.; Kang, Y.; Zhou, L.; et al. The PLAG1-GDH1 Axis Promotes Anoikis Resistance and Tumor Metastasis through CamKK2-AMPK Signaling in LKB1-Deficient Lung Cancer. Mol. Cell 2017, 69, 87–99.e7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.; Li, B.; Feng, H. PLAG1 silencing promotes cell chemosensitivity in ovarian cancer via the IGF2 signaling pathway. Int. J. Mol. Med. 2020, 45, 703–714. [Google Scholar] [CrossRef] [Green Version]
- PLAG1, the Prototype of the PLAG Gene Family: Versatility in Tumour Development (Review). Available online: https://pubmed.ncbi.nlm.nih.gov/17332914/ (accessed on 3 May 2021).
- Cruz-García, D.; Diaz-Ruiz, A.; Rabanal-Ruiz, Y.; Peinado, J.R.; Gracia-Navarro, F.; Castaño, J.P.; Montero-Hadjadje, M.; Tonon, M.; Vaudry, H.; Anouar, Y.; et al. The Golgi-associated long coiled-coil protein NECC1 participates in the control of the regulated secretory pathway in PC12 cells. Biochem. J. 2012, 443, 387–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilan, F.; Nacfer, M.; Fresquet, F.; Norez, C.; Melin, P.; Martin-Berge, A.; de Beauregard, M.-A.C.; Becq, F.; Kitzis, A.; Thoreau, V. Endosomal SNARE proteins regulate CFTR activity and trafficking in epithelial cells. Exp. Cell Res. 2008, 314, 2199–2211. [Google Scholar] [CrossRef] [PubMed]
- Murray, R.; Wylie, F.G.; Khromykh, T.; Hume, D.; Stow, J. Syntaxin 6 and Vti1b Form a Novel SNARE Complex, Which Is Up-regulated in Activated Macrophages to Facilitate Exocytosis of Tumor Necrosis Factor-α. J. Biol. Chem. 2005, 280, 10478–10483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peinado, H.; Zhang, H.; Matei, I.R.; Costa-Silva, B.; Hoshino, A.; Rodrigues, G.; Psaila, B.; Kaplan, R.N.; Bromberg, J.F.; Kang, Y.; et al. Pre-metastatic niches: Organ-specific homes for metastases. Nat. Rev. Cancer 2017, 17, 302–317. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olivan, M.; Garcia, M.; Suárez, L.; Guiu, M.; Gros, L.; Méndez, O.; Rigau, M.; Reventós, J.; Segura, M.F.; de Torres, I.; et al. Loss of microRNA-135b Enhances Bone Metastasis in Prostate Cancer and Predicts Aggressiveness in Human Prostate Samples. Cancers 2021, 13, 6202. https://doi.org/10.3390/cancers13246202
Olivan M, Garcia M, Suárez L, Guiu M, Gros L, Méndez O, Rigau M, Reventós J, Segura MF, de Torres I, et al. Loss of microRNA-135b Enhances Bone Metastasis in Prostate Cancer and Predicts Aggressiveness in Human Prostate Samples. Cancers. 2021; 13(24):6202. https://doi.org/10.3390/cancers13246202
Chicago/Turabian StyleOlivan, Mireia, Marta Garcia, Leticia Suárez, Marc Guiu, Laura Gros, Olga Méndez, Marina Rigau, Jaume Reventós, Miguel F. Segura, Inés de Torres, and et al. 2021. "Loss of microRNA-135b Enhances Bone Metastasis in Prostate Cancer and Predicts Aggressiveness in Human Prostate Samples" Cancers 13, no. 24: 6202. https://doi.org/10.3390/cancers13246202