Bridging the Species Gap: Morphological and Molecular Comparison of Feline and Human Intestinal Carcinomas
Abstract
:Simple Summary
Abstract
1. Introduction
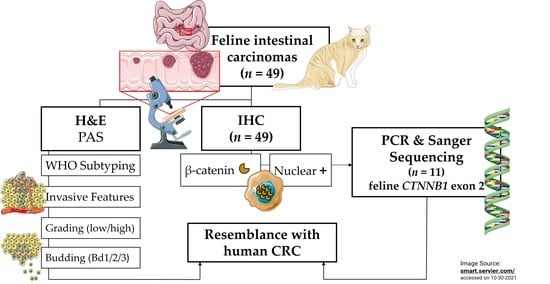
2. Materials and Methods
2.1. Feline Study Cohort
2.1.1. Tissue Processing
2.1.2. Histomorphological Characterization
2.1.3. Semiquantitative Evaluation and Computer-Assisted Image Analysis
2.1.4. Sanger Sequencing of Feline CTNNB1 Exon 2
2.2. Human Specimen
2.3. Statistical Analysis
3. Results
3.1. Tumor Site and Frequency
3.2. Distribution of Histopathological Subtypes of the Feline Intestinal Tumor Cohort
3.3. Histopathological Features of Feline Intestinal Tumor Subtypes
3.4. Immunohistochemical Features of the Feline Intestinal Tumor Cohort
3.5. β-Catenin Gene Mutations in Exon 2 of Feline CTNNB1
3.6. Comparison of Feline Small Intestinal and Colonic Neoplasias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Washington, M.K.; Powell, A.E.; Sullivan, R.; Sundberg, J.P.; Wright, N.; Coffey, R.J.; Dove, W.F. Pathology of rodent models of intestinal cancer: Progress report and recommendations. Gastroenterology 2013, 144, 705–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackstadt, R.; Sansom, O.J. Mouse models of intestinal cancer. J. Pathol. 2016, 238, 141–151. [Google Scholar] [CrossRef] [Green Version]
- Taketo, M.M.; Edelmann, W. Mouse models of colon cancer. Gastroenterology 2009, 136, 780–798. [Google Scholar] [CrossRef]
- McIntyre, R.E.; Buczacki, S.J.; Arends, M.J.; Adams, D.J. Mouse models of colorectal cancer as preclinical models. Bioessays 2015, 37, 909–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McIntyre, R.E.; van der Weyden, L.; Adams, D.J. Cancer gene discovery in the mouse. Curr. Opin. Genet. Dev. 2012, 22, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Vail, D.M.; MacEwen, E.G. Spontaneously occurring tumors of companion animals as models for human cancer. Cancer Investig. 2000, 18, 781–792. [Google Scholar] [CrossRef]
- Schwittlick, U.; Becker, S.; Aupperle-Lellbach, H. Vorkommen und Lokalisation von gastrointestinalen Neoplasien bei 293 Katzen. Kleintiermedizin 2020, 6, 250–253. [Google Scholar]
- Rissetto, K.; Villamil, J.A.; Selting, K.A.; Tyler, J.; Henry, C.J. Recent trends in feline intestinal neoplasia: An epidemiologic study of 1129 cases in the veterinary medical database from 1964 to 2004. J. Am. Anim. Hosp. Assoc. 2011, 47, 28–36. [Google Scholar] [CrossRef]
- Bonfanti, U.; Bertazzolo, W.; Bottero, E.; De Lorenzi, D.; Marconato, L.; Masserdotti, C.; Zatelli, A.; Zini, E. Diagnostic value of cytologic examination of gastrointestinal tract tumors in dogs and cats: 83 cases (2001–2004). J. Am. Vet. Med. Assoc. 2006, 229, 1130–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turk, M.A.; Gallina, A.M.; Russell, T.S. Nonhematopoietic gastrointestinal neoplasia in cats: A retrospective study of 44 cases. Vet. Pathol. 1981, 18, 614–620. [Google Scholar] [CrossRef]
- Uneyama, M.; Chambers, J.K.; Nakashima, K.; Uchida, K.; Nakayama, H. Histological Classification and Immunohistochemical Study of Feline Colorectal Epithelial Tumors. Vet. Pathol. 2021, 58, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, A.K.; Liu, S.K.; Johnson, G.F. Feline intestinal adenocarcinoma. A clinicopathologic study of 22 cases. Vet. Pathol. 1976, 13, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Manuali, E.; Forte, C.; Vichi, G.; Genovese, D.A.; Mancini, D.; De Leo, A.A.P.; Cavicchioli, L.; Pierucci, P.; Zappulli, V. Tumours in European Shorthair cats: A retrospective study of 680 cases. J. Feline Med. Surg. 2020, 22, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Slawienski, M.J.; Mauldin, G.E.; Mauldin, G.N.; Patnaik, A.K. Malignant colonic neoplasia in cats: 46 cases (1990–1996). J. Am. Vet. Med. Assoc. 1997, 211, 878–881. [Google Scholar]
- Hume, D.Z.; Solomon, J.A.; Weisse, C.W. Palliative use of a stent for colonic obstruction caused by adenocarcinoma in two cats. J. Am. Vet. Med. Assoc. 2006, 228, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Jesinghaus, M.; Schmitt, M.; Lang, C.; Reiser, M.; Scheiter, A.; Konukiewitz, B.; Steiger, K.; Silva, M.; Tschurtschenthaler, M.; Lange, S.; et al. Morphology Matters: A Critical Reappraisal of the Clinical Relevance of Morphologic Criteria From the 2019 WHO Classification in a Large Colorectal Cancer Cohort Comprising 1004 Cases. Am. J. Surg. Pathol. 2021, 45, 969–978. [Google Scholar] [CrossRef]
- Lugli, A.; Kirsch, R.; Ajioka, Y.; Bosman, F.; Cathomas, G.; Dawson, H.; El Zimaity, H.; Fléjou, J.F.; Hansen, T.P.; Hartmann, A.; et al. Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod. Pathol. 2017, 30, 1299–1311. [Google Scholar] [CrossRef]
- Nagtegaal, I.; Arends, M.; Odze, R. Tumours of the Colon and Rectum: WHO Classification of Tumours of the Colon and Rectum, TNM Staging of Carcinomas of the Colon and Rectum and the Introduction. In World Health Organization Classification of Tumours of the Digestive System; IARC Press: Geneva, Switzerland, 2019; pp. 157–187. [Google Scholar]
- Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012, 487, 330–337. [Google Scholar] [CrossRef] [Green Version]
- Arnold, A.; Tronser, M.; Sers, C.; Ahadova, A.; Endris, V.; Mamlouk, S.; Horst, D.; Möbs, M.; Bischoff, P.; Kloor, M.; et al. The majority of β-catenin mutations in colorectal cancer is homozygous. BMC Cancer 2020, 20, 1038. [Google Scholar] [CrossRef]
- Wang, J.; Wang, T.; Sun, Y.; Feng, Y.; Kisseberth, W.C.; Henry, C.J.; Mok, I.; Lana, S.E.; Dobbin, K.; Northrup, N.; et al. Proliferative and Invasive Colorectal Tumors in Pet Dogs Provide Unique Insights into Human Colorectal Cancer. Cancers 2018, 10, 330. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, R.S.; Huh, W.J.; Cates, J.M.; Washington, K.; Beauchamp, R.D.; Coffey, R.J.; Shi, C. Micropapillary colorectal carcinoma: Clinical, pathological and molecular properties, including evidence of epithelial-mesenchymal transition. Histopathology 2017, 70, 223–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meuten, D.J.; Moore, F.M.; George, J.W. Mitotic Count and the Field of View Area: Time to Standardize. Vet. Pathol. 2016, 53, 7–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Tate, J.G.; Bamford, S.; Jubb, H.C.; Sondka, Z.; Beare, D.M.; Bindal, N.; Boutselakis, H.; Cole, C.G.; Creatore, C.; Dawson, E.; et al. COSMIC: The Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2019, 47, D941–D947. [Google Scholar] [CrossRef] [Green Version]
- Salto-Tellez, M.; Rugge, M. Tumours of the Small Intestine and Ampulla. In World Health Organization Classification of Tumours of the Digestive System; Salto-Tellez, M., Nagtegaal, I., Rugge, M., Eds.; IARC Press: Geneva, Switzerland, 2019. [Google Scholar]
- Prall, F.; Maletzki, C.; Hühns, M.; Krohn, M.; Linnebacher, M. Colorectal carcinoma tumour budding and podia formation in the xenograft microenvironment. PLoS ONE 2017, 12, e0186271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georges, L.M.C.; De Wever, O.; Galván, J.A.; Dawson, H.; Lugli, A.; Demetter, P.; Zlobec, I. Cell Line Derived Xenograft Mouse Models Are a Suitable in vivo Model for Studying Tumor Budding in Colorectal Cancer. Front. Med. 2019, 6, 139. [Google Scholar] [CrossRef]
- Pai, R.K.; Bettington, M.; Srivastava, A.; Rosty, C. An update on the morphology and molecular pathology of serrated colorectal polyps and associated carcinomas. Mod. Pathol. 2019, 32, 1390–1415. [Google Scholar] [CrossRef] [PubMed]
- Jass, J.R. Classification of colorectal cancer based on correlation of clinical, morphological and molecular features. Histopathology 2007, 50, 113–130. [Google Scholar] [CrossRef]
- Rex, D.K.; Ahnen, D.J.; Baron, J.A.; Batts, K.P.; Burke, C.A.; Burt, R.W.; Goldblum, J.R.; Guillem, J.G.; Kahi, C.J.; Kalady, M.F.; et al. Serrated lesions of the colorectum: Review and recommendations from an expert panel. Am. J. Gastroenterol. 2012, 107, 1315–1329, quiz 1314, 1330. [Google Scholar] [CrossRef] [Green Version]
- Valenta, T.; Hausmann, G.; Basler, K. The many faces and functions of β-catenin. Embo. J. 2012, 31, 2714–2736. [Google Scholar] [CrossRef] [Green Version]
- Tetsu, O.; McCormick, F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 1999, 398, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Herbst, A.; Jurinovic, V.; Krebs, S.; Thieme, S.E.; Blum, H.; Göke, B.; Kolligs, F.T. Comprehensive analysis of β-catenin target genes in colorectal carcinoma cell lines with deregulated Wnt/β-catenin signaling. BMC Genom. 2014, 15, 74. [Google Scholar] [CrossRef] [Green Version]
- He, T.C.; Sparks, A.B.; Rago, C.; Hermeking, H.; Zawel, L.; da Costa, L.T.; Morin, P.J.; Vogelstein, B.; Kinzler, K.W. Identification of c-MYC as a target of the APC pathway. Science 1998, 281, 1509–1512. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Chambers, J.K.; Nakashima, K.; Uchida, E.; Ohno, K.; Tsujimoto, H.; Uchida, K.; Nakayama, H. Histopathologic Features of Colorectal Adenoma and Adenocarcinoma Developing Within Inflammatory Polyps in Miniature Dachshunds. Vet. Pathol. 2018, 55, 654–662. [Google Scholar] [CrossRef] [Green Version]
- McEntee, M.F.; Brenneman, K.A. Dysregulation of beta-catenin is common in canine sporadic colorectal tumors. Vet. Pathol. 1999, 36, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Herstad, K.M.V.; Gunnes, G.; Rørtveit, R.; Kolbjørnsen, Ø.; Tran, L.; Skancke, E. Immunohistochemical expression of β-catenin, Ki67, CD3 and CD18 in canine colorectal adenomas and adenocarcinomas. BMC Vet. Res. 2021, 17, 119. [Google Scholar] [CrossRef]
- LeBlanc, A.K.; Breen, M.; Choyke, P.; Dewhirst, M.; Fan, T.M.; Gustafson, D.L.; Helman, L.J.; Kastan, M.B.; Knapp, D.W.; Levin, W.J.; et al. Perspectives from man’s best friend: National Academy of Medicine’s Workshop on Comparative Oncology. Sci. Transl. Med. 2016, 8, 324ps325. [Google Scholar] [CrossRef] [Green Version]
- Sammarco, A.; Gomiero, C.; Sacchetto, R.; Beffagna, G.; Michieletto, S.; Orvieto, E.; Cavicchioli, L.; Gelain, M.E.; Ferro, S.; Patruno, M.; et al. Wnt/β-Catenin and Hippo Pathway Deregulation in Mammary Tumors of Humans, Dogs, and Cats. Vet. Pathol. 2020, 57, 774–790. [Google Scholar] [CrossRef]
- Suriano, G.; Vrcelj, N.; Senz, J.; Ferreira, P.; Masoudi, H.; Cox, K.; Nabais, S.; Lopes, C.; Machado, J.C.; Seruca, R.; et al. beta-catenin (CTNNB1) gene amplification: A new mechanism of protein overexpression in cancer. Genes Chromosomes Cancer 2005, 42, 238–246. [Google Scholar] [CrossRef]






| Cohort | Subtype | n | % of Total |
|---|---|---|---|
| Histological Subtypes (Overall Cohort, n = 49) | ANOS | 29 | 59.18 |
| SAC | 13 | 26.53 | |
| MAC | 5 | 10.20 | |
| MPC | 1 | 2.04 | |
| SRCC | 1 | 2.04 | |
| Histological Subtype (Small Intestinal, n = 16) | ANOS | 12 | 75.00 |
| SAC | 1 | 6.25 | |
| MAC | 2 | 12.5 | |
| MPC | 1 | 6.25 | |
| Histological Subtype (Colonic, n = 33) | ANOS | 17 | 51.52 |
| SAC | 12 | 36.36 | |
| MAC | 3 | 9.09 | |
| SRCC | 1 | 3.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Groll, T.; Schopf, F.; Denk, D.; Mogler, C.; Schwittlick, U.; Aupperle-Lellbach, H.; Sarker, S.R.J.; Pfarr, N.; Weichert, W.; Matiasek, K.; et al. Bridging the Species Gap: Morphological and Molecular Comparison of Feline and Human Intestinal Carcinomas. Cancers 2021, 13, 5941. https://doi.org/10.3390/cancers13235941
Groll T, Schopf F, Denk D, Mogler C, Schwittlick U, Aupperle-Lellbach H, Sarker SRJ, Pfarr N, Weichert W, Matiasek K, et al. Bridging the Species Gap: Morphological and Molecular Comparison of Feline and Human Intestinal Carcinomas. Cancers. 2021; 13(23):5941. https://doi.org/10.3390/cancers13235941
Chicago/Turabian StyleGroll, Tanja, Franziska Schopf, Daniela Denk, Carolin Mogler, Ulrike Schwittlick, Heike Aupperle-Lellbach, Sabrina Rim Jahan Sarker, Nicole Pfarr, Wilko Weichert, Kaspar Matiasek, and et al. 2021. "Bridging the Species Gap: Morphological and Molecular Comparison of Feline and Human Intestinal Carcinomas" Cancers 13, no. 23: 5941. https://doi.org/10.3390/cancers13235941