Heart Failure Therapies for the Prevention of HER2-Monoclonal Antibody-Mediated Cardiotoxicity: A Systematic Review and Meta-Analysis of Randomized Trials
Abstract
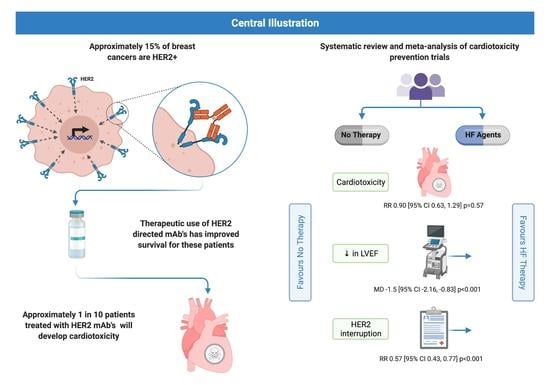
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Search Strategy
2.3. Data Collection
2.4. Data Items
2.5. Assessment of Risk of Bias in Included Studies
2.6. Data Synthesis and Analysis
2.7. Certainty Assessment
3. Results
3.1. Baseline Characteristics
3.2. Primary Outcome
3.3. Secondary Outcomes
3.3.1. HER2 Therapy Interruption
3.3.2. Change in LVEF
3.3.3. Global Longitudinal Strain
3.3.4. Serum Biomarkers
3.3.5. Adverse Events
3.3.6. Other Outcomes of Interest
4. Discussion
4.1. Limitations
4.2. Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Seshadri, R.; Matthews, C.; Dobrovic, A.; Horsfall, D.J. The Significance of Oncogene Amplification in Primary Breast Cancer. Int. J. Cancer 1989, 43, 270–272. [Google Scholar] [CrossRef]
- Press, M.; Pike, M.; Chazin, V.; Hung, G.; Udove, J.; Markowicz, M.; Danyluk, J.; Godolphin, W.; Sliwowski, M.; Akita, R.; et al. Her-2/Neu Expression in Node-Negative Breast Cancer: Direct Tissue Quantitation by Computerized Image Analysis and Association of Overexpression with Increased Risk of Recurrent Disease. Cancer Res. 1993, 53, 4960–4970. [Google Scholar] [PubMed]
- Baselga, J. Phase I and II Clinical Trials of Trastuzumab. Ann. Oncol. 2001, 12, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Perez, E.; Romond, E.; Suman, V.; Jeong, J.; Sledge, G.; Geyer, C.; Martino, S.; Rastogi, P.; Gralow, J.; Swain, S.; et al. Trastuzumab plus Adjuvant Chemotherapy for Human Epidermal Growth Factor Receptor 2-Positive Breast Cancer: Planned Joint Analysis of Overall Survival from NSABP B-31 and NCCTG N9831. J. Clin. Oncol. 2014, 32, 3744–3752. [Google Scholar] [CrossRef]
- Pivot, X.; Romieu, G.; Debled, M.; Pierga, J.-Y.; Kerbrat, P.; Bachelot, T.; Lortholary, A.; Espié, M.; Fumoleau, P.; Serin, D.; et al. 6 Months versus 12 Months of Adjuvant Trastuzumab for Patients with HER2-Positive Early Breast Cancer (PHARE): A Randomised Phase 3 Trial. Lancet Oncol. 2013, 14, 741–748. [Google Scholar] [CrossRef]
- Earl, H.M.; Hiller, L.; Vallier, A.-L.; Loi, S.; McAdam, K.; Hughes-Davies, L.; Harnett, A.N.; Ah-See, M.-L.; Simcock, R.; Rea, D.; et al. 6 versus 12 Months of Adjuvant Trastuzumab for HER2-Positive Early Breast Cancer (PERSEPHONE): 4-Year Disease-Free Survival Results of a Randomised Phase 3 Non-Inferiority Trial. Lancet 2019, 393, 2599–2612. [Google Scholar] [CrossRef] [Green Version]
- Mavroudis, D.; Saloustros, E.; Malamos, N.; Kakolyris, S.; Boukovinas, I.; Papakotoulas, P.; Kentepozidis, N.; Ziras, N.; Georgoulias, V. Six versus 12 Months of Adjuvant Trastuzumab in Combination with Dose-Dense Chemotherapy for Women with HER2-Positive Breast Cancer: A Multicenter Randomized Study by the Hellenic Oncology Research Group (HORG). Ann. Oncol. 2015, 26, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Baselga, J.; Cortés, J.; Kim, S.-B.; Im, S.-A.; Hegg, R.; Im, Y.-H.; Roman, L.; Pedrini, J.L.; Pienkowski, T.; Knott, A.; et al. Pertuzumab plus Trastuzumab plus Docetaxel for Metastatic Breast Cancer. N. Engl. J. Med. 2012, 366, 109–119. [Google Scholar] [CrossRef] [Green Version]
- Fedele, C.; Riccio, G.; Malara, A.; D’Alessio, G.; de Lorenzo, C. Mechanisms of Cardiotoxicity Associated with ErbB2 Inhibitors. Breast Cancer Res. Treat. 2012, 134, 595–602. [Google Scholar] [CrossRef]
- De Keulenaer, G.; Doggen, K.; Lemmens, K. The Vulnerability of the Heart as a Pluricellular Paracrine Organ: Lessons from Unexpected Triggers of Heart Failure in Targeted ErbB2 Anticancer Therapy. Circ. Res. 2010, 106, 35–46. [Google Scholar] [CrossRef] [Green Version]
- Maurea, N.; Coppola, C.; Piscopo, G.; Galletta, F.; Riccio, G.; Esposito, E.; de Lorenzo, C.; de Laurentiis, M.; Spallarossa, P.; Mercuro, G. Pathophysiology of Cardiotoxicity from Target Therapy and Angiogenesis Inhibitors. J. Cardiovasc. Med. 2016, 17, S19–S26. [Google Scholar] [CrossRef]
- Lotrionte, M.; Biondi-Zoccai, G.; Abbate, A.; Lanzetta, G.; D’Ascenzo, F.; Malavasi, V.; Peruzzi, M.; Frati, G.; Palazzoni, G. Review and Meta-Analysis of Incidence and Clinical Predictors of Anthracycline Cardiotoxicity. Am. J. Cardiol. 2013, 112, 1980–1984. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.A.; Cornelius, V.R.; Plummer, C.J.; Levitt, G.; Verrill, M.; Canney, P.; Jones, A. Cardiotoxicity of Anthracycline Agents for the Treatment of Cancer: Systematic Review and Meta-Analysis of Randomised Controlled Trials. BMC Cancer 2010, 10, 337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seidman, A.; Hudis, C.; Pierri, M.K.; Shak, S.; Paton, V.; Ashby, M.; Murphy, M.; Stewart, S.J.; Keefe, D. Cardiac Dysfunction in the Trastuzumab Clinical Trials Experience. J. Clin. Oncol. 2016, 20, 1215–1221. [Google Scholar] [CrossRef]
- Chavez-MacGregor, M.; Zhang, N.; Buchholz, T.; Zhang, Y.; Niu, J.; Elting, L.; Smith, B.; Hortobagyi, G.; Giordano, S. Trastuzumab-Related Cardiotoxicity among Older Patients with Breast Cancer. J. Clin. Oncol. 2013, 31, 4222–4228. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E. Early Breast Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Copeland-Halperin, R.; Al-Sadawi, M.; Patil, S.; Liu, J.; Steingart, R.; Dang, C.; Yu, A. Early Trastuzumab Interruption and Recurrence-Free Survival in ERBB2-Positive Breast Cancer. JAMA Oncol. 2020, 6, 1971–1972. [Google Scholar] [CrossRef]
- Joensuu, H.; Fraser, J.; Wildiers, H.; Huovinen, R.; Auvinen, P.; Utriainen, M.; Nyandoto, P.; Villman, K.K.; Halonen, P.; Granstam-Björneklett, H.; et al. Effect of Adjuvant Trastuzumab for a Duration of 9 Weeks vs 1 Year With Concomitant Chemotherapy for Early Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer: The SOLD Randomized Clinical Trial. JAMA Oncol. 2018, 4, 1199–1206. [Google Scholar] [CrossRef]
- Chen, J.; Colosimo, M.; Lim, E. The Management of HER2-positive Early Breast Cancer: Current and Future Therapies. Asia-Pac. J. Clin. Oncol. 2021, 17, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Tajiri, K.; Aonuma, K.; Sekine, I. Cardio-Oncology: A Multidisciplinary Approach for Detection, Prevention and Management of Cardiac Dysfunction in Cancer Patients. Jpn. J. Clin. Oncol. 2017, 47, 678–682. [Google Scholar] [CrossRef] [Green Version]
- Kostakou, P.; Kouris, N.; Kostopoulos, V.; Damaskos, D.; Olympios, C. Cardio-Oncology: A New and Developing Sector of Research and Therapy in the Field of Cardiology. Heart Fail. Rev. 2019, 24, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Yancy, C.W.; Mariell Jessup, C.; Chair Biykem Bozkurt, V.; Butler, J.; Casey, D.E., Jr.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017, 136, 137–161. [Google Scholar] [CrossRef]
- Zamorano, J.L.; Lancellotti, P.; Rodriguez Muñoz, D.; Aboyans, V.; Asteggiano, R.; Galderisi, M.; Habib, G.; Lenihan, D.J.; Lip, G.Y.H.; Lyon, A.R.; et al. 2016 ESC Position Paper on Cancer Treatments and Cardiovascular Toxicity Developed under the Auspices of the ESC Committee for Practice Guidelines. Eur. Heart J. 2016, 37, 2768–2801. [Google Scholar] [CrossRef]
- Barac, A.; Blaes, A.; Lynce, F. Lessons From Primary Cardiac Prevention Trials During Trastuzumab Therapy. J. Am. Coll. Cardiol. 2019, 73, 2869–2871. [Google Scholar] [CrossRef]
- Padegimas, A.; Clasen, S.; Ky, B. Cardioprotective Strategies to Prevent Breast Cancer Therapy-Induced Cardiotoxicity. Trends Cardiovasc. Med. 2020, 30, 22–28. [Google Scholar] [CrossRef]
- Blanter, J.B.; Frishman, W.H. The Preventive Role of Angiotensin Converting Enzyme Inhibitors/Angiotensin-II Receptor Blockers and β-Adrenergic Blockers in Anthracycline- and Trastuzumab-Induced Cardiotoxicity. Cardiol. Rev. 2019, 27, 256–259. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [Green Version]
- Luo, D.; Wan, X.; Liu, J.; Tong, T. Optimally Estimating the Sample Mean from the Sample Size, Median, Mid-Range, and/or Mid-Quartile Range. Stat. Methods Med. Res. 2018, 27, 1785–1805. [Google Scholar] [CrossRef] [Green Version]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the Sample Mean and Standard Deviation from the Sample Size, Median, Range and/or Interquartile Range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V. Cochrane Handbook for Systematic Reviews of Interventions Version 6.2. Available online: www.training.cochrane.org/handbook (accessed on 1 August 2021).
- GRADE Working Group. Grading Quality of Evidence and Strength of Recommendations. BMJ 2004, 328, 1490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherafati, A.; Mehrpooya, M.; Badkoubeh, R.S.; Larti, F.; Shahi, F.; Mirzania, M.; Esfandbod, M.; Saadat, M.; Ghasemi, M.; Zebardast, J. Assessment of Carvedilol Therapy in Prevention of Heart Failure in HER2 Positive Breast Cancer Patients Receiving Trastuzumab. Acta Medica Iranica 2019, 57, 173–179. [Google Scholar] [CrossRef]
- Pituskin, E.; Mackey, J.R.; Koshman, S.; Jassal, D.; Pitz, M.; Haykowsky, M.J.; Pagano, J.J.; Chow, K.; Thompson, R.B.; Vos, L.J.; et al. Multidisciplinary Approach to Novel Therapies in Cardio-Oncology Research (MANTICORE 101–Breast): A Randomized Trial for the Prevention of Trastuzumab-Associated Cardiotoxicity. J. Clin. Oncol. 2016, 35, 870–877. [Google Scholar] [CrossRef]
- Boekhout, A.H.; Gietema, J.A.; Kerklaan, B.M.; van Werkhoven, E.D.; Altena, R.; Honkoop, A.; Los, M.; Smit, W.M.; Nieboer, P.; Smorenburg, C.H.; et al. Angiotensin II–Receptor Inhibition With Candesartan to Prevent Trastuzumab-Related Cardiotoxic Effects in Patients With Early Breast Cancer: A Randomized Clinical Trial. JAMA Oncol. 2016, 2, 1030–1037. [Google Scholar] [CrossRef]
- Guglin, M.; Krischer, J.; Tamura, R.; Fink, A.; Bello-Matricaria, L.; McCaskill-Stevens, W.; Munster, P.N. Randomized Trial of Lisinopril Versus Carvedilol to Prevent Trastuzumab Cardiotoxicity in Patients With Breast Cancer. J. Am. Coll. Cardiol. 2019, 73, 2859–2868. [Google Scholar] [CrossRef]
- Farahani, M.; Nourian, S.; Jalalian, H.R.; Khosravi, A.; Salesi, M. Efficacy of Treatment With Carvedilol in Preventing Early-Stage Left Ventricular Dysfunction in Patients With Breast Cancer Candidated to Receive Trastuzumab Using 2D Speckle-Tracking Echocardiography. Iran. Heart J. 2019, 20, 20–31. [Google Scholar]
- Stoodley, P.W.; Richards, D.A.B.; Hui, R.; Boyd, A.; Harnett, P.R.; Meikle, S.R.; Clarke, J.; Thomas, L. Two-Dimensional Myocardial Strain Imaging Detects Changes in Left Ventricular Systolic Function Immediately after Anthracycline Chemotherapy. Eur. J. Echocardiogr. 2011, 12, 945–952. [Google Scholar] [CrossRef] [Green Version]
- Thavendiranathan, P.; Negishi, T.; Somerset, E.; Negishi, K.; Penicka, M.; Lemieux, J.; Aakhus, S.; Miyazaki, S.; Shirazi, M.; Galderisi, M.; et al. Strain-Guided Management of Potentially Cardiotoxic Cancer Therapy. J. Am. Coll. Cardiol. 2021, 77, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.; Colombo, A.; Torrisi, R.; Sandri, M.T.; Civelli, M.; Salvatici, M.; Lamantia, G.; Colombo, N.; Cortinovis, S.; Dessanai, M.A.; et al. Trastuzumab-Induced Cardiotoxicity: Clinical and Prognostic Implications of Troponin I Evaluation. J. Clin. Oncol. 2010, 28, 3910–3916. [Google Scholar] [CrossRef]
- Ewer, M.S.; Ewer, S.M. Troponin I Provides Insight Into Cardiotoxicity and the Anthracycline-Trastuzumab Interaction. J. Clin. Oncol. 2010, 28, 3901–3904. [Google Scholar] [CrossRef]
- Zardavas, D.; Suter, T.M.; van Veldhuisen, D.J.; Steinseifer, J.; Noe, J.; Lauer, S.; Al-Sakaff, N.; Piccart-Gebhart, M.J.; de Azambuja, E. Role of Troponins I and T and N -Terminal Prohormone of Brain Natriuretic Peptide in Monitoring Cardiac Safety of Patients With Early-Stage Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer Receiving Trastuzumab: A Herceptin Adjuvant Study Cardiac Marker Substudy. J. Clin. Oncol. 2017, 35, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Ponde, N.; Bradbury, I.; Lambertini, M.; Ewer, M.; Campbell, C.; Ameels, H.; Zardavas, D.; di Cosimo, S.; Baselga, J.; Huober, J.; et al. Cardiac Biomarkers for Early Detection and Prediction of Trastuzumab and/or Lapatinib-Induced Cardiotoxicity in Patients with HER2-Positive Early-Stage Breast Cancer: A NeoALTTO Sub-Study (BIG 1-06). Breast Cancer Res. Treat. 2018, 168, 631–638. [Google Scholar] [CrossRef]
- Elkhateeb, A.M.; Diab, D.R.; Ibrahim, H.; Gado, N.; Ali, Y.A.; Ibrahim, A.S. Prevention of Cardiotoxicity in Breast Cancer Patients: A Randomized Prospective Study. Ann. Oncol. 2017, 28, v64. [Google Scholar] [CrossRef]
- Heck, S.L.; Mecinaj, A.; Ree, A.H.; Hoffmann, P.; Schulz-Menger, J.; Fagerland, M.W.; Gravdehaug, B.; Røsjø, H.; Steine, K.; Geisler, J.; et al. Prevention of Cardiac Dysfunction During Adjuvant Breast Cancer Therapy (PRADA): Extended Follow-Up of a 2 × 2 Factorial, Randomized, Placebo-Controlled, Double-Blind Clinical Trial of Candesartan and Metoprolol. Circulation 2021, 143, 2431–2440. [Google Scholar] [CrossRef]
- Livi, L.; Barletta, G.; Martella, F.; Desideri, I.; Scotti, V.; Becherini, C.; Saieva, C.; Terziani, F.; Bacci, C.; Airoldi, M.; et al. Pre-Specified Interim Analysis of the SAFE Trial (NCT2236806): A 4-Arm Randomized, Double-Blind, Controlled Study Evaluating the Efficacy and Safety of Cardiotoxicity Prevention in Non-Metastatic Breast Cancer Patients Treated with Anthracyclines with or without Trastuzumab. Ann. Oncol. 2019, 30, v72. [Google Scholar] [CrossRef]
- Cohn, J.N.; Archibald, D.G.; Ziesche, S.; Franciosa, J.A.; Harston, W.E.; Tristani, F.E.; Dunkman, W.B.; Jacobs, W.; Francis, G.S.; Flohr, K.H.; et al. Effect of Vasodilator Therapy on Mortality in Chronic Congestive Heart Failure. N. Engl. J. Med. 2009, 41, 269–271. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure. N. Engl. J. Med. 2014, 5, 132–133. [Google Scholar] [CrossRef] [Green Version]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 13, 17–18. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Qadir, H.; Ong, G.; Fazelzad, R.; Amir, E.; Lee, D.S.; Thavendiranathan, P.; Tomlinson, G. Interventions for Preventing Cardiomyopathy Due to Anthracyclines: A Bayesian Network Meta-Analysis. Ann. Oncol. 2017, 28, 628–633. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lam, C.S.P.; Chandramouli, C.; Ahooja, V.; Verma, S. SGLT-2 Inhibitors in Heart Failure: Current Management, Unmet Needs, and Therapeutic Prospects. J. Am. Heart Assoc. 2019, 8, e013389. [Google Scholar] [CrossRef] [PubMed]
- Thavendiranathan, P.; Abdel-Qadir, H.; Fischer, H.; Camacho, X.; Amir, E.; Austin, P.; Lee, D. Breast Cancer Therapy-Related Cardiac Dysfunction in Adult Women Treated in Routine Clinical Practice: A Population-Based Cohort Study. J. Clin. Oncol. 2016, 34, 2239–2246. [Google Scholar] [CrossRef] [PubMed]





| Study | n | Study Design | Age, Mean +/− SD (Years) | Early or Metastatic | Anthracyclines | Type of Intervention | Intervention | Comparator | Follow Up | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Boekhout et al. [36] | 206 | Double-blind RCT | 49 +/− 24.1 | Early | All | ARB | Candesartan 32 mg/day | Placebo | 21 months |
|
| Farahani et al. [38] | 71 | Single-blind, open-label RCT | 57 +/− 8.8 | Early | All | Beta-blocker | Carvedilol 6.25 mg BD to MTD | No treatment | 12 weeks |
|
| Guglin et al. [37] | 468 | Double-blind RCT | 51 +/− 10.93 | Early | 189 of 468 (40.1%) | ACE-inhibitor OR Beta-blocker | Lisinopril 10 mg daily OR Carvedilol 10 mg daily | Placebo | 52 weeks |
|
| Pituskin et al. [35] | 94 | Double-blind RCT | 50 +/− 10 | Early | 22 of 94 (23.4%) | ACE-inhibitor OR Beta-blocker | Perindopril 2 mg daily OR bisoprolol 2.5 mg daily titrated to MTD | Placebo | 52 weeks |
|
| Sherafati et al. [34] | 65 | Double-blind RCT | 46.5 | Early/Metastatic | NR | Beta-blocker | Carvedilol 6.25 mg BD | No treatment | 12 weeks |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brown, L.J.; Meredith, T.; Yu, J.; Patel, A.; Neal, B.; Arnott, C.; Lim, E. Heart Failure Therapies for the Prevention of HER2-Monoclonal Antibody-Mediated Cardiotoxicity: A Systematic Review and Meta-Analysis of Randomized Trials. Cancers 2021, 13, 5527. https://doi.org/10.3390/cancers13215527
Brown LJ, Meredith T, Yu J, Patel A, Neal B, Arnott C, Lim E. Heart Failure Therapies for the Prevention of HER2-Monoclonal Antibody-Mediated Cardiotoxicity: A Systematic Review and Meta-Analysis of Randomized Trials. Cancers. 2021; 13(21):5527. https://doi.org/10.3390/cancers13215527
Chicago/Turabian StyleBrown, Lauren J., Thomas Meredith, Jie Yu, Anushka Patel, Bruce Neal, Clare Arnott, and Elgene Lim. 2021. "Heart Failure Therapies for the Prevention of HER2-Monoclonal Antibody-Mediated Cardiotoxicity: A Systematic Review and Meta-Analysis of Randomized Trials" Cancers 13, no. 21: 5527. https://doi.org/10.3390/cancers13215527