Sex Differences in Overall Survival and the Effect of Radiotherapy in Merkel Cell Carcinoma—A Retrospective Analysis of A Swedish Cohort
Abstract
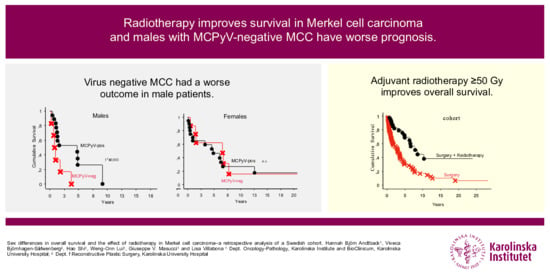
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Cohort Characteristics and Overall Survival
2.2. MCPyV-Status and Overall Survival
2.3. Treatment and Overall Survival
3. Discussion
4. Materials and Methods
4.1. Patient Selection
4.2. Surgery
4.3. Radiotherapy Treatment
4.4. McPyV Analysis
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paulson, K.G.; Park, S.Y.; Vandeven, N.A.; Lachance, K.; Thomas, H.; Chapuis, A.G.; Harms, K.L.; Thompson, J.A.; Bhatia, S.; Stang, A.; et al. Merkel cell carcinoma: Current US incidence and projected increases based on changing demographics. J. Am. Acad. Dermatol. 2018, 78, 457–463.e2. [Google Scholar] [CrossRef]
- Stang, A.; Becker, J.C.; Nghiem, P.; Ferlay, J. The association between geographic location and incidence of Merkel cell carcinoma in comparison to melanoma: An international assessment. Eur. J. Cancer 2018, 94, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Bjornhagen, V. Nationellt Vårdprogram för Merkelcellscancer. 2020. [Updated 16 November 2020]. Available online: https://www.cancercentrum.se/globalassets/vara-uppdrag/kunskapsstyrning/vardprogram/kommande-vardprogram/2020/200915/nvp-merkelcellscancer-2020-09-15.pdf (accessed on 6 January 2011).
- Lemos, B.; Nghiem, P. Merkel cell carcinoma: More deaths but still no pathway to blame. J. Investig. Dermatol. 2007, 127, 2100–2103. [Google Scholar] [CrossRef] [PubMed]
- Agelli, M.; Clegg, L.X. Epidemiology of primary Merkel cell carcinoma in the United States. J. Am. Acad. Dermatol. 2003, 49, 832–841. [Google Scholar] [CrossRef]
- Zaar, O.; Gillstedt, M.; Lindelof, B.; Wennberg-Larko, A.M.; Paoli, J. Merkel cell carcinoma incidence is increasing in Sweden. J. Eur. Acad. Dermatol. Venereol. 2016, 30, 1708–1713. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, N.C. Merkel cell carcinoma: Changing incidence trends. J. Surg. Oncol. 2005, 89, 1–4. [Google Scholar] [CrossRef]
- Feng, H.; Shuda, M.; Chang, Y.; Moore, P.S. Clonal integration of a polyomavirus in human Merkel cell carcinoma. Science 2008, 319, 1096–1100. [Google Scholar] [CrossRef] [Green Version]
- Pietropaolo, V.; Prezioso, C.; Moens, U. Merkel Cell Polyomavirus and Merkel Cell Carcinoma. Cancers 2020, 12, 1774. [Google Scholar] [CrossRef]
- Higaki-Mori, H.; Kuwamoto, S.; Iwasaki, T.; Kato, M.; Murakami, I.; Nagata, K.; Sano, H.; Horie, Y.; Yoshida, Y.; Yamamoto, O.; et al. Association of Merkel cell polyomavirus infection with clinicopathological differences in Merkel cell carcinoma. Hum. Pathol. 2012, 43, 2282–2291. [Google Scholar] [CrossRef]
- Sihto, H.; Kukko, H.; Koljonen, V.; Sankila, R.; Bohling, T.; Joensuu, H. Merkel cell polyomavirus infection, large T antigen, retinoblastoma protein and outcome in Merkel cell carcinoma. Clin. Cancer Res. 2011, 17, 4806–4813. [Google Scholar] [CrossRef] [Green Version]
- Moshiri, A.S.; Doumani, R.; Yelistratova, L.; Blom, A.; Lachance, K.; Shinohara, M.M.; Delaney, M.; Chang, O.; McArdle, S.; Thomas, H.; et al. Polyomavirus-Negative Merkel Cell Carcinoma: A More Aggressive Subtype Based on Analysis of 282 Cases Using Multimodal Tumor Virus Detection. J. Investig. Dermatol. 2017, 137, 819–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reichgelt, B.A.; Visser, O. Epidemiology and survival of Merkel cell carcinoma in the Netherlands. A population-based study of 808 cases in 1993–2007. Eur. J. Cancer 2011, 47, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Kukko, H.; Bohling, T.; Koljonen, V.; Tukiainen, E.; Haglund, C.; Pokhrel, A.; Sankila, R.; Pukkala, E. Merkel cell carcinoma—A population-based epidemiological study in Finland with a clinical series of 181 cases. Eur. J. Cancer 2012, 48, 737–742. [Google Scholar] [CrossRef] [PubMed]
- Tam, M.; Luu, M.; Barker, C.A.; Gharavi, N.M.; Hamid, O.; Shiao, S.L.; Nguyen, A.T.; Lu, D.J.; Ho, A.S.; Zumsteg, Z.S. Improved survival in women versus men with merkel cell carcinoma. J. Am. Acad. Dermatol. 2020. [Google Scholar] [CrossRef]
- Heath, M.; Jaimes, N.; Lemos, B.; Mostaghimi, A.; Wang, L.C.; Penas, P.F.; Nghiem, P. Clinical characteristics of Merkel cell carcinoma at diagnosis in 195 patients: The AEIOU features. J. Am. Acad. Dermatol. 2008, 58, 375–381. [Google Scholar] [CrossRef] [Green Version]
- Albores-Saavedra, J.; Batich, K.; Chable-Montero, F.; Sagy, N.; Schwartz, A.M.; Henson, D.E. Merkel cell carcinoma demographics, morphology, and survival based on 3870 cases: A population based study. J. Cutan. Pathol. 2010, 37, 20–27. [Google Scholar] [CrossRef]
- Petrelli, F.; Ghidini, A.; Torchio, M.; Prinzi, N.; Trevisan, F.; Dallera, P.; De Stefani, A.; Russo, A.; Vitali, E.; Bruschieri, L.; et al. Adjuvant radiotherapy for Merkel cell carcinoma: A systematic review and meta-analysis. Radiother. Oncol. 2019, 134, 211–219. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Russell, J.S.; Hamid, O.; Bhatia, S.; Terheyden, P.; D’Angelo, S.P.; Shih, K.C.; Lebbe, C.; Milella, M.; Brownell, I.; et al. Updated efficacy of avelumab in patients with previously treated metastatic Merkel cell carcinoma after ≥1 year of follow-up: JAVELIN Merkel 200, a phase 2 clinical trial. J. Immunother. Cancer 2018, 6, 7. [Google Scholar] [CrossRef] [Green Version]
- Nghiem, P.T.; Bhatia, S.; Lipson, E.J.; Kudchadkar, R.R.; Miller, N.J.; Annamalai, L.; Berry, S.; Chartash, E.K.; Daud, A.; Fling, S.P.; et al. PD-1 Blockade with Pembrolizumab in Advanced Merkel-Cell Carcinoma. N. Engl. J. Med. 2016, 374, 2542–2552. [Google Scholar] [CrossRef]
- Knepper, T.C.; Montesion, M.; Russell, J.S.; Sokol, E.S.; Frampton, G.M.; Miller, V.A.; Albacker, L.A.; McLeod, H.L.; Eroglu, Z.; Khushalani, N.I.; et al. The Genomic Landscape of Merkel Cell Carcinoma and Clinicogenomic Biomarkers of Response to Immune Checkpoint Inhibitor Therapy. Clin. Cancer Res. 2019, 25, 5961–5971. [Google Scholar] [CrossRef] [Green Version]
- Engels, E.A.; Frisch, M.; Goedert, J.J.; Biggar, R.J.; Miller, R.W. Merkel cell carcinoma and HIV infection. Lancet 2002, 359, 497–498. [Google Scholar] [CrossRef] [Green Version]
- Lanoy, E.; Engels, E.A. Skin cancers associated with autoimmune conditions among elderly adults. Br. J. Cancer 2010, 103, 112–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, C.A.; Robbins, H.A.; Tatalovich, Z.; Lynch, C.F.; Pawlish, K.S.; Finch, J.L.; Hernandez, B.Y.; Fraumeni, J.F., Jr.; Madeleine, M.M.; Engels, E.A. Risk of merkel cell carcinoma after solid organ transplantation. J. Natl. Cancer Inst. 2015, 107, dju382. [Google Scholar] [CrossRef] [Green Version]
- Harrington, C.; Kwan, W. Radiotherapy and Conservative Surgery in the Locoregional Management of Merkel Cell Carcinoma: The British Columbia Cancer Agency Experience. Ann. Surg. Oncol. 2016, 23, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Finnigan, R.; Hruby, G.; Wratten, C.; Keller, J.; Tripcony, L.; Dickie, G.; Rischin, D.; Poulsen, M. The impact of preradiation residual disease volume on time to locoregional failure in cutaneous Merkel cell carcinoma--a TROG substudy. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Strom, T.; Carr, M.; Zager, J.S.; Naghavi, A.; Smith, F.O.; Cruse, C.W.; Messina, J.L.; Russell, J.; Rao, N.G.; Fulp, W.; et al. Radiation Therapy is Associated with Improved Outcomes in Merkel Cell Carcinoma. Ann. Surg. Oncol. 2016, 23, 3572–3578. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.M.; Roman, S.A.; Sosa, J.A.; Judson, B.L. The role of adjuvant therapy in the management of head and neck merkel cell carcinoma: An analysis of 4815 patients. JAMA Otolaryngol. Head Neck Surg. 2015, 141, 137–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatia, S.; Storer, B.E.; Iyer, J.G.; Moshiri, A.; Parvathaneni, U.; Byrd, D.; Sober, A.J.; Sondak, V.K.; Gershenwald, J.E.; Nghiem, P. Adjuvant Radiation Therapy and Chemotherapy in Merkel Cell Carcinoma: Survival Analyses of 6908 Cases From the National Cancer Data Base. J. Natl. Cancer Inst. 2016, 108, djw042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatia, K.; Goedert, J.J.; Modali, R.; Preiss, L.; Ayers, L.W. Merkel cell carcinoma subgroups by Merkel cell polyomavirus DNA relative abundance and oncogene expression. Int. J. Cancer 2010, 126, 2240–2246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Handschel, J.; Muller, D.; Depprich, R.A.; Ommerborn, M.A.; Kubler, N.R.; Naujoks, C.; Reifenberger, J.; Schafer, K.L.; Braunstein, S. The new polyomavirus (MCPyV) does not affect the clinical course in MCCs. Int. J. Oral Maxillofac. Surg. 2010, 39, 1086–1090. [Google Scholar] [CrossRef]
- Nardi, V.; Song, Y.; Santamaria-Barria, J.A.; Cosper, A.K.; Lam, Q.; Faber, A.C.; Boland, G.M.; Yeap, B.Y.; Bergethon, K.; Scialabba, V.L.; et al. Activation of PI3K signaling in Merkel cell carcinoma. Clin. Cancer Res. 2012, 18, 1227–1236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laude, H.C.; Jonchere, B.; Maubec, E.; Carlotti, A.; Marinho, E.; Couturaud, B.; Peter, M.; Sastre-Garau, X.; Avril, M.F.; Dupin, N.; et al. Distinct merkel cell polyomavirus molecular features in tumour and non tumour specimens from patients with merkel cell carcinoma. PLoS Pathog. 2010, 6, e1001076. [Google Scholar] [CrossRef]
- Harms, K.L.; Healy, M.A.; Nghiem, P.; Sober, A.J.; Johnson, T.M.; Bichakjian, C.K.; Wong, S.L. Analysis of Prognostic Factors from 9387 Merkel Cell Carcinoma Cases Forms the Basis for the New 8th Edition AJCC Staging System. Ann. Surg. Oncol. 2016, 23, 3564–3571. [Google Scholar] [CrossRef] [PubMed]
- Rodig, S.J.; Cheng, J.; Wardzala, J.; DoRosario, A.; Scanlon, J.J.; Laga, A.C.; Martinez-Fernandez, A.; Barletta, J.A.; Bellizzi, A.M.; Sadasivam, S.; et al. Improved detection suggests all Merkel cell carcinomas harbor Merkel polyomavirus. J. Clin. Investig. 2012, 122, 4645–4653. [Google Scholar] [CrossRef] [Green Version]
- Botticelli, A.; Onesti, C.E.; Zizzari, I.; Cerbelli, B.; Sciattella, P.; Occhipinti, M.; Roberto, M.; Di Pietro, F.; Bonifacino, A.; Ghidini, M.; et al. The sexist behaviour of immune checkpoint inhibitors in cancer therapy? Oncotarget 2017, 8, 99336–99346. [Google Scholar] [CrossRef]
- Wu, Y.; Ju, Q.; Jia, K.; Yu, J.; Shi, H.; Wu, H.; Jiang, M. Correlation between sex and efficacy of immune checkpoint inhibitors (PD-1 and CTLA-4 inhibitors). Int. J. Cancer 2018, 143, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Conforti, F.; Pala, L.; Goldhirsch, A. Different effectiveness of anticancer immunotherapy in men and women relies on sex-dimorphism of the immune system. Oncotarget 2018, 9, 31167–31168. [Google Scholar] [CrossRef] [PubMed]
- Grassadonia, A.; Sperduti, I.; Vici, P.; Iezzi, L.; Brocco, D.; Gamucci, T.; Pizzuti, L.; Maugeri-Sacca, M.; Marchetti, P.; Cognetti, G.; et al. Effect of Gender on the Outcome of Patients Receiving Immune Checkpoint Inhibitors for Advanced Cancer: A Systematic Review and Meta-Analysis of Phase III Randomized Clinical Trials. J. Clin. Med. 2018, 7, 542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahi, H.; Their, J.; Gissler, M.; Koljonen, V. Merkel Cell Carcinoma Treatment in Finland in 1986–2016—A Real-World Data Study. Cancers 2020, 12, 1224. [Google Scholar] [CrossRef] [PubMed]
- Asgari, M.M.; Sokil, M.M.; Warton, E.M.; Iyer, J.; Paulson, K.G.; Nghiem, P. Effect of host, tumor, diagnostic, and treatment variables on outcomes in a large cohort with Merkel cell carcinoma. JAMA Dermatol. 2014, 150, 716–723. [Google Scholar] [CrossRef] [Green Version]
- Xie, H.; Lee, L.; Caramuta, S.; Hoog, A.; Browaldh, N.; Bjornhagen, V.; Larsson, C.; Lui, W.O. MicroRNA expression patterns related to merkel cell polyomavirus infection in human merkel cell carcinoma. J. Investig. Dermatol. 2014, 134, 507–517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, H.; Liu, T.; Wang, N.; Bjornhagen, V.; Hoog, A.; Larsson, C.; Lui, W.O.; Xu, D. TERT promoter mutations and gene amplification: Promoting TERT expression in Merkel cell carcinoma. Oncotarget 2014, 5, 10048–10057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Xie, H.; Shi, H.; Gao, J.; Juhlin, C.C.; Bjornhagen, V.; Hoog, A.; Lee, L.; Larsson, C.; Lui, W.O. Merkel cell polyomavirus oncoproteins induce microRNAs that suppress multiple autophagy genes. Int. J. Cancer 2020, 146, 1652–1666. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Cohort Characteristics | Cohort | Female | Male | ||||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Cohort | 113 | 100 | 64 | 57 | 49 | 43 | |
| Age | Median, years | 76 | 79 | 75 | |||
| 19–69 | 25 | 22 | 18 | 28 | 7 | 14 | |
| >70 | 88 | 78 | 46 | 46 | 42 | 86 | |
| Tumor Location | Head and neck | 53 | 47 | 30 | 47 | 23 | 47 |
| Upper extremity | 24 | 21 | 14 | 22 | 10 | 20 | |
| Lower extremity | 20 | 18 | 13 | 20 | 7 | 14 | |
| Trunk | 12 | 11 | 5 | 8 | 7 | 14 | |
| Genital area | 4 | 4 | 2 | 3 | 2 | 4 | |
| Stage | I | 64 | 57 | 36 | 56 | 28 | 57 |
| II | 35 | 31 | 22 | 34 | 13 | 27 | |
| III | 14 | 12 | 6 | 9 | 8 | 16 | |
| MCPyV-Status in Tumor | 54 | 29 | 25 | ||||
| Positive | 40 | 74 | 21 | 72 | 19 | 76 | |
| Negative | 14 | 26 | 8 | 28 | 6 | 24 | |
| Treatment | Surgery | 66 | 58 | 36 | 56 | 30 | 61 |
| Surgery and radiotherapy | 47 | 42 | 28 | 44 | 19 | 39 | |
| Parameters | Extremities vs. Trunk | Extremities vs. H&N | H&N vs. Trunk | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Cohort | Females | Males | Cohort | Females | Males | Cohort | Females | Males | |
| Hazard | 0.48 | 0.35 | 0.88 | 0.53 | 0.52 | 0.48 | 0.9 | 0.65 | 1.6 |
| C.I. 95% | 0.23–0.97 | 0.12–1.02 | 0.31–2.4 | 0.32–0.87 | 0.24–1.11 | 0.21–1.08 | 0.47–1.7 | 0.24–1.7 | 0.65–4.3 |
| P | 0.03 | 0.05 | ns | 0.034 | ns | ns | ns | ns | ns |
| Sample | MCPyV Negative vs. Positive | ||
|---|---|---|---|
| HR | 95% C.I. | p-Value | |
| MCPyV cohort (n = 54) | 1.3 | 0.65–2.6 | ns |
| Females (n = 29) | 0.84 | 0.32–2.2 | ns |
| Males (n = 25) | 3.6 | 1.2–10 | 0.018 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Björn Andtback, H.; Björnhagen-Säfwenberg, V.; Shi, H.; Lui, W.-O.; Masucci, G.V.; Villabona, L. Sex Differences in Overall Survival and the Effect of Radiotherapy in Merkel Cell Carcinoma—A Retrospective Analysis of A Swedish Cohort. Cancers 2021, 13, 265. https://doi.org/10.3390/cancers13020265
Björn Andtback H, Björnhagen-Säfwenberg V, Shi H, Lui W-O, Masucci GV, Villabona L. Sex Differences in Overall Survival and the Effect of Radiotherapy in Merkel Cell Carcinoma—A Retrospective Analysis of A Swedish Cohort. Cancers. 2021; 13(2):265. https://doi.org/10.3390/cancers13020265
Chicago/Turabian StyleBjörn Andtback, Hannah, Viveca Björnhagen-Säfwenberg, Hao Shi, Weng-Onn Lui, Giuseppe V. Masucci, and Lisa Villabona. 2021. "Sex Differences in Overall Survival and the Effect of Radiotherapy in Merkel Cell Carcinoma—A Retrospective Analysis of A Swedish Cohort" Cancers 13, no. 2: 265. https://doi.org/10.3390/cancers13020265