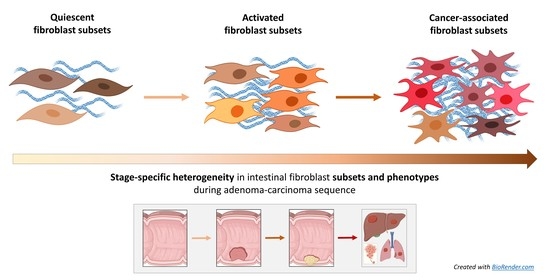
Fibroblast Subsets in Intestinal Homeostasis, Carcinogenesis, Tumor Progression, and Metastasis
Abstract
:Simple Summary
Abstract
1. Introduction and Definitions
2. Fibroblasts in Intestinal Homeostasis
2.1. Fibroblast Subsets in Normal Intestinal Mucosa
2.2. Fibroblasts and Intestinal Adenoma Formation
3. Fibroblasts in Intestinal Carcinogenesis
3.1. Adenomas
3.2. Early-Invasive Colorectal Cancers
4. Fibroblasts in Advanced CRC Progression and Metastasis
4.1. Tumor Growth, Invasion, and TME Remodeling
4.2. Therapeutic Resistance and Immune Regulation
4.3. Metastasis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Arnold, M.; Abnet, C.C.; Neale, R.E.; Vignat, J.; Giovannucci, E.L.; McGlynn, K.A.; Bray, F. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology 2020, 159, 335–349.e15. [Google Scholar] [CrossRef] [PubMed]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [Green Version]
- Kuipers, E.J.; Grady, W.M.; Lieberman, D.; Seufferlein, T.; Sung, J.J.; Boelens, P.G.; van de Velde, C.J.; Watanabe, T. Colorectal cancer. Nat. Rev. Dis. Primers 2015, 1, 15065. [Google Scholar] [CrossRef] [Green Version]
- Fearon, E.R.; Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 1990, 61, 759–767. [Google Scholar] [CrossRef]
- Kalluri, R. The biology and function of fibroblasts in cancer. Nat. Rev. Cancer 2016, 16, 582–598. [Google Scholar] [CrossRef]
- Kobayashi, H.; Enomoto, A.; Woods, S.L.; Burt, A.D.; Takahashi, M.; Worthley, D.L. Cancer-associated fibroblasts in gastrointestinal cancer. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 282–295. [Google Scholar] [CrossRef]
- Mesker, W.E.; Junggeburt, J.M.C.; Szuhai, K.; De Heer, P.; Morreau, H.; Tanke, H.J.; Tollenaar, R.A. The Carcinoma–Stromal Ratio of Colon Carcinoma Is an Independent Factor for Survival Compared to Lymph Node Status and Tumor Stage. Cell. Oncol. 2007, 29, 387–398. [Google Scholar]
- Huijbers, A.; Tollenaar, R.A.; Pelt, G.W.V.; Zeestraten, E.C.M.; Dutton, S.; McConkey, C.C.; Domingo, E.; Smit, V.T.H.B.M.; Midgley, R.; Warren, B.F.; et al. The proportion of tumor-stroma as a strong prognosticator for stage II and III colon cancer patients: Validation in the VICTOR trial. Ann. Oncol. 2013, 24, 179–185. [Google Scholar] [CrossRef]
- Guinney, J.; Dienstmann, R.; Wang, X.; De Reyniès, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P.; et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef]
- Calon, A.; Lonardo, E.; Berenguer-Llergo, A.; Espinet, E.; Hernando-Momblona, X.; Iglesias, M.; Sevillano, M.; Palomo-Ponce, S.; Tauriello, D.V.; Byrom, D.; et al. Stromal gene expression defines poor-prognosis subtypes in colorectal cancer. Nat. Genet. 2015, 47, 320–329. [Google Scholar] [CrossRef] [Green Version]
- Isella, C.; Terrasi, A.; Bellomo, S.E.; Petti, C.; Galatola, G.; Muratore, A.; Mellano, A.; Senetta, R.; Cassenti, A.; Sonetto, C.; et al. Stromal contribution to the colorectal cancer transcriptome. Nat. Genet. 2015, 47, 312–319. [Google Scholar] [CrossRef]
- Dunne, P.D.; McArt, D.G.; Bradley, C.A.; O’Reilly, P.G.; Barrett, H.L.; Cummins, R.; O’Grady, A.; Arthur, K.; Loughrey, M.B.; Allen, W.L.; et al. Challenging the Cancer Molecular Stratification Dogma: Intratumoral Heterogeneity Undermines Consensus Molecular Subtypes and Potential Diagnostic Value in Colorectal Cancer. Clin. Cancer Res. 2016, 22, 4095–4104. [Google Scholar] [CrossRef] [Green Version]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T.; et al. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef] [Green Version]
- Gieniec, K.A.; Butler, L.M.; Worthley, D.L.; Woods, S.L. Cancer-associated fibroblasts—heroes or villains? Br. J. Cancer 2019, 121, 293–302. [Google Scholar] [CrossRef]
- Fridman, W.H.; Miller, I.S.; Sautès-Fridman, C.; Byrne, A.T. Therapeutic Targeting of the Colorectal Tumor Stroma. Gastroenterology 2020, 158, 303–321. [Google Scholar] [CrossRef]
- Barnhoorn, M.C.; Hakuno, S.K.; Bruckner, R.S.; Rogler, G.; Hawinkels, L.J.A.C.; Scharl, M. Stromal Cells in the Pathogenesis of Inflammatory Bowel Disease. J. Crohn’s Colitis 2020, 14, 995–1009. [Google Scholar] [CrossRef]
- Roulis, M.; Flavell, R.A. Fibroblasts and myofibroblasts of the intestinal lamina propria in physiology and disease. Differentiation 2016, 92, 116–131. [Google Scholar] [CrossRef]
- Pinchuk, I.V.; Mifflin, R.C.; Saada, J.I.; Powell, D.W. Intestinal mesenchymal cells. Curr. Gastroenterol. Rep. 2010, 12, 310–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lockwood, D.S.; Yeadon, T.M.; Clouston, A.D.; Crawford, D.G.; Fawcett, J.; Callaghan, S.A.; Gotley, D.C. Tumor progression in hepato-cellular carcinoma: Relationship with tumor stroma and parenchymal disease. J. Gastroenterol. Hepatol. 2003, 18, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Abbas, O.; Mahalingam, M. Desmoplasia: Not always a bad thing. Histopathology 2010, 58, 643–659. [Google Scholar] [CrossRef] [PubMed]
- Paulsson, J.; Micke, P. Prognostic relevance of cancer-associated fibroblasts in human cancer. Semin. Cancer Biol. 2014, 25, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Rieder, F.; Brenmoehl, J.; Leeb, S.; Scholmerich, J.; Rogler, G. Wound healing and fibrosis in intestinal disease. Gut 2007, 56, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, H.F. Tumors: Wounds that do not heal. Similarities between tumor stroma generation and wound healing. N. Engl. J. Med. 1986, 315, 1650–1659. [Google Scholar] [PubMed]
- Kretzschmar, K.; Weber, C.; Driskell, R.R.; Calonje, E.; Watt, F.M. Compartmentalized Epidermal Activation of beta-Catenin Differ-entially Affects Lineage Reprogramming and Underlies Tumor Heterogeneity. Cell Rep. 2016, 14, 269–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arina, A.; Idel, C.; Hyjek, E.M.; Alegre, M.-L.; Wang, Y.; Bindokas, V.P.; Weichselbaum, R.R.; Schreiber, H. Tumor-associated fibroblasts predominantly come from local and not circulating precursors. Proc. Natl. Acad. Sci. USA 2016, 113, 7551–7556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powell, D.W.; Pinchuk, I.V.; Saada, J.I.; Chen, X.; Mifflin, R.C. Mesenchymal cells of the intestinal lamina propria. Annu. Rev. Physiol. 2011, 73, 213–237. [Google Scholar] [CrossRef] [Green Version]
- Lawrance, I.C.; Rogler, G.; Bamias, G.; Breynaert, C.; Florholmen, J.; Pellino, G.; Reif, S.; Speca, S.; Latella, G. Cellular and Molecular Me-diators of Intestinal Fibrosis. J. Crohn’s Colitis 2017, 11, 1491–1503. [Google Scholar]
- Powell, D.W.; Mifflin, R.; Valentich, J.D.; Crowe, S.E.; Saada, J.; West, A.B. Myofibroblasts. II. Intestinal subepithelial myofibroblasts. Am. J. Physiol. Content 1999, 277, C183–C201. [Google Scholar] [CrossRef]
- Degirmenci, B.; Valenta, T.; Dimitrieva, S.; Hausmann, G.; Basler, K. GLI1-expressing mesenchymal cells form the essential Wnt-secreting niche for colon stem cells. Nat. Cell Biol. 2018, 558, 449–453. [Google Scholar] [CrossRef]
- Valenta, T.; Degirmenci, B.; Moor, A.E.; Herr, P.; Zimmerli, D.; Moor, M.B.; Hausmann, G.; Cantù, C.; Aguet, M.; Basler, K. Wnt Ligands Secreted by Subepithelial Mesenchymal Cells Are Essential for the Survival of Intestinal Stem Cells and Gut Homeostasis. Cell Rep. 2016, 15, 911–918. [Google Scholar] [CrossRef] [Green Version]
- Nowarski, R.; Jackson, R.; Flavell, R.A. The Stromal Intervention: Regulation of Immunity and Inflammation at the Epitheli-al-Mesenchymal Barrier. Cell 2017, 168, 362–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navin, N.; Kendall, J.; Troge, J.; Andrews, P.; Rodgers, L.; McIndoo, J.; Cook, K.; Stepansky, A.; Levy, D.; Esposito, D.; et al. Tumour evo-lution inferred by single-cell sequencing. Nature 2011, 472, 90–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinchen, J.; Chen, H.H.; Parikh, K.; Antanaviciute, A.; Jagielowicz, M.; Fawkner-Corbett, D.; Ashley, N.; Cubitt, L.; Mellado-Gomez, E.; Attar, M.; et al. Structural Remodeling of the Human Colonic Mesenchyme in Inflammatory Bowel Disease. Cell 2018, 175, 372–386.e17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nik, A.M.; Reyahi, A.; Pontén, F.; Carlsson, P. Foxf2 in Intestinal Fibroblasts Reduces Numbers of Lgr5+ Stem Cells and Adenoma Formation by Inhibiting Wnt Signaling. Gastroenterology 2013, 144, 1001–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Normand, S.; Delanoye-Crespin, A.; Bressenot, A.; Huot, L.; Grandjean, T.; Peyrin-Biroulet, L.; Lemoine, Y.; Hot, D.; Chamaillard, M. Nod-like receptor pyrin domain-containing protein 6 (NLRP6) controls epithelial self-renewal and colorectal carcinogenesis upon injury. Proc. Natl. Acad. Sci. USA 2011, 108, 9601–9606. [Google Scholar] [CrossRef] [Green Version]
- Pallangyo, C.K.; Ziegler, P.K.; Greten, F.R. IKKbeta acts as a tumor suppressor in cancer-associated fibroblasts during intestinal tumorigenesis. J. Exp. Med. 2015, 212, 2253–2266. [Google Scholar] [CrossRef] [Green Version]
- Koliaraki, V.; Roulis, M.; Kollias, G. Tpl2 regulates intestinal myofibroblast HGF release to suppress colitis-associated tumorigene-sis. J. Clin. Investig. 2012, 122, 4231–4242. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.; Wang, J.; Zhao, X.; Wu, T.; Huang, Z.; Chen, D.; Liu, Y.; Ouyang, G. Periostin Promotes Colorectal Tumorigenesis through Integrin-FAK-Src Pathway-Mediated YAP/TAZ Activation. Cell Rep. 2020, 30, 793–806.e6. [Google Scholar] [CrossRef] [Green Version]
- Owusu, B.Y.; Galemmo, R.; Janetka, J.; Klampfer, L. Hepatocyte Growth Factor, a Key Tumor-Promoting Factor in the Tumor Mi-croenvironment. Cancers 2017, 9, 35. [Google Scholar] [CrossRef] [Green Version]
- Joosten, S.P.J.; Zeilstra, J.; van Andel, H.; Mijnals, R.C.; Zaunbrecher, J.; Duivenvoorden, A.A.M.; van de Wetering, M.; Clevers, H.; Spaargaren, M.; Pals, S.T. MET Signaling Mediates Intestinal Crypt-Villus Development, Regeneration, and Adenoma For-mation and Is Promoted by Stem Cell CD44 Isoforms. Gastroenterology 2017, 153, 1040–1053.e1044. [Google Scholar] [CrossRef] [Green Version]
- Vermeulen, L.; Melo, F.D.S.E.; Van Der Heijden, M.; Cameron, K.A.; De Jong, J.H.; Borovski, T.; Tuynman, J.B.; Todaro, M.; Merz, C.; Rodermond, H.M.; et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat. Cell Biol. 2010, 12, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H.; Loh, K.M.; Nusse, R. An integral program for tissue renewal and regeneration: Wnt signaling and stem cell control. Science 2014, 346, 1248012. [Google Scholar] [CrossRef] [PubMed]
- Nusse, R.; Clevers, H. Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef]
- Reya, T.; Clevers, H. Wnt signalling in stem cells and cancer. Nat. Cell Biol. 2005, 434, 843–850. [Google Scholar] [CrossRef] [PubMed]
- Allaire, J.M.; Roy, S.A.; Ouellet, C.; Lemieux, É.; Jones, C.; Paquet, M.; Boudreau, F.; Perreault, N. Bmp signaling in colonic mesenchyme regulates stromal microenvironment and protects from polyposis initiation. Int. J. Cancer 2016, 138, 2700–2712. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Ayers, J.L.; Carter, K.T.; Wang, T.; Maden, S.K.; Edmond, D.; Newcomb, P.P.; Li, C.; Ulrich, C.; Yu, M.; et al. Senescence-associated tissue microenvironment promotes colon cancer formation through the secretory factor GDF15. Aging Cell 2019, 18, e13013. [Google Scholar] [CrossRef] [Green Version]
- Roulis, M.; Kaklamanos, A.; Schernthanner, M.; Bielecki, P.; Zhao, J.; Kaffe, E.; Frommelt, L.-S.; Qu, R.; Knapp, M.S.; Henriques, A.; et al. Paracrine orchestration of intestinal tumorigenesis by a mesenchymal niche. Nat. Cell Biol. 2020, 580, 524–529. [Google Scholar] [CrossRef]
- Rostom, A.; Dube, C.; Lewin, G.; Tsertsvadze, A.; Barrowman, N.; Code, C.; Sampson, M.; Moher, D.; U.S. Preventive Services Task Force. Nonsteroidal an-ti-inflammatory drugs and cyclooxygenase-2 inhibitors for primary prevention of colorectal cancer: A systematic review pre-pared for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2007, 146, 376–389. [Google Scholar] [CrossRef]
- Hong, D.S.; Parikh, A.; Shapiro, G.I.; Varga, A.; Naing, A.; Meric-Bernstam, F.; Ataman, Ö.; Reyderman, L.; Binder, T.A.; Ren, M.; et al. First-in-human phase I study of immunomodulatory E7046, an antagonist of PGE2-receptor E-type 4 (EP4), in patients with advanced cancers. J. Immunother. Cancer 2020, 8, e000222. [Google Scholar] [CrossRef]
- Büller, N.V.; Rosekrans, S.L.; Metcalfe, C.; Heijmans, J.; Van Dop, W.A.; Fessler, E.; Jansen, M.; Ahn, C.; Vermeulen, J.L.; Westendorp, B.F.; et al. Stromal Indian Hedgehog Signaling Is Required for Intestinal Adenoma Formation in Mice. Gastroenterology 2015, 148, 170–180.e6. [Google Scholar] [CrossRef] [Green Version]
- Fujishita, T.; Kajino-Sakamoto, R.; Kojima, Y.; Taketo, M.M.; Aoki, M. Antitumor activity of the MEK inhibitor trametinib on intesti-nal polyp formation in Apc(Delta716) mice involves stromal COX-2. Cancer Sci. 2015, 106, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Uenoyama, Y.; Seno, H.; Fukuda, A.; Sekikawa, A.; Nanakin, A.; Sawabu, T.; Kawada, M.; Kanda, N.; Suzuki, K.; Yada, N.; et al. Hypox-ia induced by benign intestinal epithelial cells is associated with cyclooxygenase-2 expression in stromal cells through AP-1-dependent pathway. Oncogene 2006, 25, 3277–3285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dvorak, H.F.; Form, D.M.; Manseau, E.J.; Smith, B.D. Pathogenesis of desmoplasia. I. Immunofluorescence identification and locali-zation of some structural proteins of line 1 and line 10 guinea pig tumors and of healing wounds. J. Natl. Cancer Inst. 1984, 73, 1195–1205. [Google Scholar] [PubMed]
- Ronnov-Jessen, L.; Petersen, O.W.; Bissell, M.J. Cellular changes involved in conversion of normal to malignant breast: Importance of the stromal reaction. Physiol. Rev. 1996, 76, 69–125. [Google Scholar] [CrossRef]
- Garcia, P.E.; Adoumie, M.; Kim, E.C.; Zhang, Y.; Scales, M.K.; El-Tawil, Y.S.; Shaikh, A.Z.; Wen, H.-J.; Bednar, F.; Allen, B.L.; et al. Differential Contribution of Pancreatic Fibroblast Subsets to the Pancreatic Cancer Stroma. Cell. Mol. Gastroenterol. Hepatol. 2020, 10, 581–599. [Google Scholar] [CrossRef]
- SanGiovanni, A.; Del Ninno, E.; Fasani, P.; De Fazio, C.; Ronchi, G.; Romeo, R.; Morabito, A.; De Franchis, R.; Colombo, M. Increased survival of cirrhotic patients with a hepatocellular carcinoma detected during surveillance ☆. Gastroenterology 2004, 126, 1005–1014. [Google Scholar] [CrossRef]
- Wang, H.M.; Hung, C.H.; Lu, S.N.; Chen, C.H.; Lee, C.M.; Hu, T.H.; Wang, J.H. Liver stiffness measurement as an alternative to fibrotic stage in risk assessment of hepatocellular carcinoma incidence for chronic hepatitis C patients. Liver Int. 2013, 33, 756–761. [Google Scholar] [CrossRef]
- Li, J.; Yang, M.; Li, P.; Su, Z.; Gao, P.; Zhang, J. Idiopathic pulmonary fibrosis will increase the risk of lung cancer. Chin. Med. J. 2014, 127, 3142–3149. [Google Scholar]
- Tandon, M.; Coudriet, G.M.; Criscimanna, A.; Socorro, M.; Eliliwi, M.; Singhi, A.D.; Cruz-Monserrate, Z.; Bailey, P.; Lotze, M.T.; Zeh, H.; et al. Prolactin Promotes Fibrosis and Pancreatic Cancer Progression. Cancer Res. 2019, 79, 5316–5327. [Google Scholar] [CrossRef] [Green Version]
- Özdemir, B.C.; Pentcheva-Hoang, T.; Carstens, J.L.; Zheng, X.; Wu, C.-C.; Simpson, T.R.; Laklai, H.; Sugimoto, H.; Kahlert, C.; Novitskiy, S.V.; et al. Depletion of Carcinoma-Associated Fibroblasts and Fibrosis Induces Immunosuppression and Accelerates Pancreas Cancer with Reduced Survival. Cancer Cell 2014, 25, 719–734. [Google Scholar] [CrossRef] [Green Version]
- Valkenburg, K.C.; De Groot, A.E.; Pienta, K.J. Targeting the tumour stroma to improve cancer therapy. Nat. Rev. Clin. Oncol. 2018, 15, 366–381. [Google Scholar] [CrossRef] [PubMed]
- Fessler, E.; Drost, J.; van Hooff, S.R.; Linnekamp, J.F.; Wang, X.; Jansen, M.; De Sousa, E.M.F.; Prasetyanti, P.R.; Je, I.J.; Franitza, M.; et al. TGFbeta signaling directs serrated adenomas to the mesenchymal colorectal cancer subtype. EMBO Mol. Med. 2016, 8, 745–760. [Google Scholar] [CrossRef] [PubMed]
- Calon, A.; Espinet, E.; Palomo-Ponce, S.; Tauriello, D.V.; Iglesias, M.; Cespedes, M.V.; Sevillano, M.; Nadal, C.; Jung, P.; Zhang, X.H.; et al. Dependency of colorectal cancer on a TGF-beta-driven program in stromal cells for metastasis initiation. Cancer Cell 2012, 22, 571–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glentis, A.; Oertle, P.; Mariani, P.; Chikina, A.; El Marjou, F.; Attieh, Y.; Zaccarini, F.; Lae, M.; Loew, D.; Dingli, F.; et al. Cancer-associated fibroblasts induce metalloprotease-independent cancer cell invasion of the basement membrane. Nat. Commun. 2017, 8, 1–13. [Google Scholar] [CrossRef]
- Kaye, G.I.; Pascal, R.R.; Lane, N. The colonic pericryptal fibroblast sheath: Replication, migration, and cytodifferentiation of a mesenchymal cell system in adult tissue. 3. Replication and differentiation in human hyperplastic and adenomatous polyps. Gastroenterology 1971, 60, 515–536. [Google Scholar] [CrossRef]
- Li, A.; Hasui, K.; Yonezawa, S.; Tanaka, S.; Sato, E. Immunohistochemical analysis of pericryptal fibroblast sheath and proliferating epithelial cells in human colorectal adenomas and carcinomas with adenoma components. Pathol. Int. 1999, 49, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Yao, T.; Tsuneyoshi, M. Significance of pericryptal fibroblasts in colorectal epithelial tumors: A special reference to the histologic features and growth patterns. Hum. Pathol. 1993, 24, 525–533. [Google Scholar] [CrossRef]
- Yao, T.; Tada, S.; Tsuneyoshi, M. Colorectal counterpart of gastric depressed adenoma. A comparison with flat and polypoid adenomas with special reference to the development of pericryptal fibroblasts. Am. J. Surg. Pathol. 1994, 18, 559–568. [Google Scholar] [CrossRef]
- Shimoda, T.; Ikegami, M.; Fujisaki, J.; Matsui, T.; Aizawa, S.; Ishikawa, E. Early colorectal carcinoma with special reference to its development de novo. Cancer 1989, 64, 1138–1146. [Google Scholar] [CrossRef]
- Harada, K.; Higaki, S.; Amano, A.; Hashimoto, K.; Hashimoto, S.; Gondo, T.; Sakaida, I. A reduced COX-2 expression and a reduced number of pericryptal myofibroblasts are associated with depressed adenoma of the colon. Oncol. Rep. 2007, 17, 1353–1358. [Google Scholar] [CrossRef]
- Arnoletti, J.P.; Upson, J.; Babb, J.S.; Bellacosa, A.; Watson, J.C. Differential stromal and epithelial localization of cyclooxygenase-2 (COX-2) during colorectal tumorigenesis. J. Exp. Clin. Cancer Res. 2005, 24, 279–287. [Google Scholar] [PubMed]
- Kikuchi, Y.; Kashima, T.G.; Nishiyama, T.; Shimazu, K.; Morishita, Y.; Shimazaki, M.; Kii, I.; Horie, H.; Nagai, H.; Kudo, A.; et al. Periostin Is Expressed in Pericryptal Fibroblasts and Cancer-associated Fibroblasts in the Colon. J. Histochem. Cytochem. 2008, 56, 753–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedmann, Y.; Vlodavsky, I.; Aingorn, H.; Aviv, A.; Peretz, T.; Pecker, I.; Pappo, O. Expression of Heparanase in Normal, Dysplastic, and Neoplastic Human Colonic Mucosa and Stroma. Am. J. Pathol. 2000, 157, 1167–1175. [Google Scholar] [CrossRef]
- Tzelepi, V.; Grivas, P.; Kefalopoulou, Z.; Kalofonos, H.; Varakis, J.N.; Sotiropoulou-Bonikou, G. Expression of estrogen receptor co-regulators NCoR and PELP1 in epithelial cells and myofibroblasts of colorectal carcinomas: Cytoplasmic translocation of NCoR in epithelial cells correlates with better [corrected] prognosis. Virchows Arch. 2009, 454, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Tzelepi, V.; Grivas, P.; Kefalopoulou, Z.; Kalofonos, H.; Varakis, J.N.; Melachrinou, M.; Sotiropoulou-Bonikou, G. Estrogen signaling in colorectal carcinoma microenvironment: Expression of ERbeta1, AIB-1, and TIF-2 is upregulated in cancer-associated myofi-broblasts and correlates with disease progression. Virchows Arch. 2009, 454, 389–399. [Google Scholar] [CrossRef]
- Sordat, I.; Chaubert, P.; Protiva, P.; Guillou, L.; Mazzucchelli, L.; Saraga, E.; Benhattar, J.; Trân-Thang, C.; Blum, A.L.; Dorta, G.; et al. In situ stromal expression of the urokinase/plasmin system correlates with epithelial dysplasia in colorectal adenomas. Am. J. Pathol. 1997, 150, 283–295. [Google Scholar]
- Ogawa, H.; Iwaya, K.; Izumi, M.; Kuroda, M.; Serizawa, H.; Koyanagi, Y.; Mukai, K. Expression of CD10 by stromal cells during colo-rectal tumor development. Hum. Pathol. 2002, 33, 806–811. [Google Scholar] [CrossRef]
- Nakayama, H.; Enzan, H.; Yasui, W. Expression of podoplanin/D2-40 in pericryptal stromal cells in superficial colorectal epitheli-al neoplasia. Med. Mol. Morphol. 2013, 46, 20–23. [Google Scholar] [CrossRef]
- Yamanashi, T.; Nakanishi, Y.; Fujii, G.; Akishima-Fukasawa, Y.; Moriya, Y.; Kanai, Y.; Watanabe, M.; Hirohashi, S. Podoplanin expres-sion identified in stromal fibroblasts as a favorable prognostic marker in patients with colorectal carcinoma. Oncology 2009, 77, 53–62. [Google Scholar] [CrossRef]
- Choi, S.-Y.; Sung, R.; Lee, S.-J.; Lee, T.-G.; Kim, N.; Yoon, S.M.; Lee, E.J.; Chae, H.B.; Youn, S.J.; Park, S.M. Podoplanin, α-Smooth Muscle Actin or S100A4 Expressing Cancer-Associated Fibroblasts Are Associated with Different Prognosis in Colorectal Cancers. J. Korean Med. Sci. 2013, 28, 1293–1301. [Google Scholar] [CrossRef] [Green Version]
- Cui, L.; Ohuchida, K.; Mizumoto, K.; Moriyama, T.; Onimaru, M.; Nakata, K.; Nabae, T.; Ueki, T.; Sato, N.; Tominaga, Y.; et al. Prospec-tively isolated cancer-associated CD10(+) fibroblasts have stronger interactions with CD133(+) colon cancer cells than with CD133(-) cancer cells. PLoS ONE 2010, 5, e12121. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Chen, J.; Yao, H.; Liu, J.; Yu, S.; Lao, L.; Wang, M.; Luo, M.; Xing, Y.; Chen, F.; et al. CD10(+)GPR77(+) Cancer-Associated Fibro-blasts Promote Cancer Formation and Chemoresistance by Sustaining Cancer Stemness. Cell 2018, 172, 841–856.e816. [Google Scholar] [CrossRef]
- Zhu, Y.; Zheng, J.J.; Yang, F.; Nie, Q.Q.; Zhu, Z.L.; Deng, H. Expression of CD10 in cancer-associated fibroblasts and its effect on initi-ation and progression of colorectal carcinoma. Zhonghua Bing Li Xue Za Zhi 2016, 45, 859–865. [Google Scholar] [PubMed]
- Behrens, P.; Mathiak, M.; Mangold, E.; Kirdorf, S.; Wellmann, A.; Fogt, F.; Rothe, M.; Florin, A.; Wernert, N. Stromal expression of invasion-promoting, matrix-degrading proteases MMP-1 and -9 and the Ets 1 transcription factor in HNPCC carcinomas and sporadic colorectal cancers. Int. J. Cancer 2003, 107, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Roncucci, L.; Sena, P.; Mariani, F.; Marzona, L.; Benincasa, M.; De Leon, M.P.; Palumbo, C. Matrix metalloproteinases 15 and 19 are stromal regulators of colorectal cancer development from the early stages. Int. J. Oncol. 2012, 41, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Fleming, M.; Ravula, S.; Tatishchev, S.F.; Wang, H.L. Colorectal carcinoma: Pathologic aspects. J. Gastrointest. Oncol. 2012, 3, 153–173. [Google Scholar] [PubMed]
- Yamamoto, S.; Watanabe, M.; Hasegawa, H.; Baba, H.; Yoshinare, K.; Shiraishi, J.; Kitajima, M. The risk of lymph node metastasis in T1 colorectal carcinoma. Hepatogastroenterology 2004, 51, 998–1000. [Google Scholar] [PubMed]
- Son, H.J.; Song, S.Y.; Lee, W.Y.; Yang, S.S.; Park, S.H.; Yang, M.H.; Yoon, S.H.; Chun, H. Characteristics of early colorectal carcinoma with lymph node metastatic disease. Hepatogastroenterology 2008, 55, 1293–1297. [Google Scholar]
- Ha, R.K.; Han, K.S.; Sohn, D.K.; Kim, B.C.; Hong, C.W.; Chang, H.J.; Hyun, J.H.; Kim, M.J.; Park, S.C.; Oh, J.H. Histopathologic risk factors for lymph node metastasis in patients with T1 colorectal cancer. Ann. Surg. Treat. Res. 2017, 93, 266–271. [Google Scholar] [CrossRef] [Green Version]
- Bosch, S.L.; Teerenstra, S.; De Wilt, J.H.W.; Cunningham, C.; Nagtegaal, I.D. Predicting lymph node metastasis in pT1 colorectal cancer: A systematic review of risk factors providing rationale for therapy decisions. Endoscopy 2013, 45, 827–841. [Google Scholar] [CrossRef] [Green Version]
- Hashiguchi, Y.; Muro, K.; Saito, Y.; Ito, Y.; Ajioka, Y.; Hamaguchi, T.; Hasegawa, K.; Hotta, K.; Ishida, H.; Ishiguro, M.; et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for the treatment of colorectal cancer. Int. J. Clin. Oncol. 2020, 25, 1–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rex, D.K.; Shaukat, A.; Wallace, M.B. Optimal Management of Malignant Polyps, From Endoscopic Assessment and Resection to Decisions about Surgery. Clin. Gastroenterol. Hepatol. 2019, 17, 1428–1437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugai, T.; Uesugi, N.; Kitada, Y.; Yamada, N.; Osakabe, M.; Eizuka, M.; Sugimoto, R.; Fujita, Y.; Kawasaki, K.; Yamamoto, E.; et al. Analy-sis of the expression of cancer-associated fibroblast- and EMT-related proteins in submucosal invasive colorectal cancer. J. Cancer 2018, 9, 2702–2712. [Google Scholar] [CrossRef] [PubMed]
- Haasnoot, K.J.C.; Backes, Y.; Moons, L.M.G.; Kranenburg, O.; Trinh, A.; Vermeulen, L.; Noe, M.; Tuynman, J.B.; van Lent, A.U.G.; van Ginneken, R.; et al. Associations of non-pedunculated T1 colorectal adenocarcinoma outcome with consensus molecular sub-types, immunoscore, and microsatellite status: A multicenter case-cohort study. Mod. Pathol. 2020, 33, 2626–2636. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.; van Pelt, G.W.; Haasnoot, K.J.; Backes, Y.; Elias, S.G.; Seerden, T.C.; Schwartz, M.P.; Spanier, B.W.; de Vos Tot Nederveen Cappel, W.H.; van Bergeijk, J.D.; et al. Tumour-stroma ratio has poor prognostic value in non-pedunculated T1 colorectal can-cer: A multi-centre case-cohort study. United Eur. Gastroenterol. J. 2020, 2050640620975324. [Google Scholar] [CrossRef]
- Koliaraki, V.; Pallangyo, C.K.; Greten, F.R.; Kollias, G. Mesenchymal Cells in Colon Cancer. Gastroenterology 2017, 152, 964–979. [Google Scholar] [CrossRef] [Green Version]
- Musa, M.; Ali, A. Cancer-associated fibroblasts of colorectal cancer and their markers: Updates, challenges and translational outlook. Futur. Oncol. 2020, 16, 2329–2344. [Google Scholar] [CrossRef]
- Herrera, M.; Islam, A.B.; Herrera, A.; Martín, P.; García, V.; Silva, J.; Garcia, J.M.; Salas, C.; Casal, I.; De Herreros, A.G.; et al. Functional Heterogeneity of Cancer-Associated Fibroblasts from Human Colon Tumors Shows Specific Prognostic Gene Expression Signature. Clin. Cancer Res. 2013, 19, 5914–5926. [Google Scholar] [CrossRef] [Green Version]
- Mrazek, A.A.; Carmical, J.R.; Wood, T.G.; Hellmich, M.R.; Eltorky, M.; Bohanon, F.J.; Chao, C. Colorectal Cancer-Associated Fibroblasts are Genotypically Distinct. Curr. Cancer Ther. Rev. 2014, 10, 97–218. [Google Scholar] [CrossRef] [Green Version]
- Nishishita, R.; Morohashi, S.; Seino, H.; Wu, Y.; Yoshizawa, T.; Haga, T.; Saito, K.; Hakamada, K.; Fukuda, S.; Kijima, H. Expression of cancer-associated fibroblast markers in advanced colorectal cancer. Oncol. Lett. 2018, 15, 6195–6202. [Google Scholar] [CrossRef] [Green Version]
- Son, G.M.; Kwon, M.-S.; Shin, D.-H.; Shin, N.; Ryu, D.; Kang, C.-D. Comparisons of cancer-associated fibroblasts in the intratumoral stroma and invasive front in colorectal cancer. Medicine 2019, 98, e15164. [Google Scholar] [CrossRef] [PubMed]
- Berdiel-Acer, M.; Sanz-Pamplona, R.; Calon, A.; Cuadras, D.; Berenguer, A.; Sanjuan, X.; Paules, M.J.; Salazar, R.; Moreno, V.; Batlle, E.; et al. Differences between CAFs and their paired NCF from adjacent colonic mucosa reveal functional heterogeneity of CAFs, providing prognostic information. Mol. Oncol. 2014, 8, 1290–1305. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Courtois, E.T.; Sengupta, D.; Tan, Y.; Chen, K.H.; Goh, J.J.L.; Kong, S.L.; Chua, C.; Hon, L.K.; Tan, W.S.; et al. Reference component analysis of single-cell transcriptomes elucidates cellular heterogeneity in human colorectal tumors. Nat. Genet. 2017, 49, 708–718. [Google Scholar] [CrossRef] [PubMed]
- Brennen, W.N.; Isaacs, J.T.; Denmeade, S.R. Rationale behind targeting fibroblast activation protein-expressing carcino-ma-associated fibroblasts as a novel chemotherapeutic strategy. Mol. Cancer Ther. 2012, 11, 257–266. [Google Scholar] [CrossRef] [Green Version]
- Kakarla, S.; Song, X.-T.; Gottschalk, S. Cancer-associated fibroblasts as targets for immunotherapy. Immunotherapy 2012, 4, 1129–1138. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Zhou, L.; Li, D.; Andl, T.; Zhang, Y. Cancer-Associated Fibroblasts Build and Secure the Tumor Microenvironment. Front. Cell Dev. Biol. 2019, 7, 60. [Google Scholar] [CrossRef] [Green Version]
- Santi, A.; Kugeratski, F.G.; Zanivan, S. Cancer Associated Fibroblasts: The Architects of Stroma Remodeling. Proteomics 2018, 18, e1700167. [Google Scholar] [CrossRef]
- De Palma, M.; Biziato, D.; Petrova, T.V. Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer 2017, 17, 457–474. [Google Scholar] [CrossRef]
- Omoto, T.; Kim-Kaneyama, J.R.; Lei, X.F.; Orimo, A.; Ohnishi, K.; Yoshihara, K.; Miyauchi, A.; Li, S.; Gao, L.; Umemoto, T.; et al. The im-pact of stromal Hic-5 on the tumorigenesis of colorectal cancer through lysyl oxidase induction and stromal remodeling. Oncogene 2018, 37, 1205–1219. [Google Scholar] [CrossRef]
- Herrera, A.; Herrera, M.; Castellon, L.A.; Silva, J.; García, V.; Loubat-Casanovas, J.; Álvarez-Cienfuegos, A.; García, J.M.; Rodríguez, R.; Gil, B.; et al. Protumorigenic effects of Snail-expression fibroblasts on colon cancer cells. Int. J. Cancer 2014, 134, 2984–2990. [Google Scholar] [CrossRef] [Green Version]
- Bai, Y.P.; Shang, K.; Chen, H.; Ding, F.; Wang, Z.; Liang, C.; Xu, Y.; Sun, M.H.; Li, Y.Y. FGF-1/-3/FGFR4 signaling in cancer-associated fibroblasts promotes tumor progression in colon cancer through Erk and MMP-7. Cancer Sci. 2015, 106, 1278–1287. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Lu, H.; Qian, Z. Matrine reduces the secretion of exosomal circSLC7A6 from cancer-associated fibroblast to inhibit tu-morigenesis of colorectal cancer by regulating CXCR5. Biochem. Biophys. Res. Commun. 2020, 527, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Henriksson, M.L.; Edin, S.; Dahlin, A.M.; Oldenborg, P.-A.; Öberg, Å.; Van Guelpen, B.; Rutegård, J.; Stenling, R.; Palmqvist, R. Colorectal Cancer Cells Activate Adjacent Fibroblasts Resulting in FGF1/FGFR3 Signaling and Increased Invasion. Am. J. Pathol. 2011, 178, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, T.; Karasawa, H.; Funayama, R.; Shirota, M.; Suzuki, T.; Maeda, S.; Suzuki, H.; Yamamura, A.; Naitoh, T.; Nakayama, K.; et al. Cancer-associated fibroblasts secrete Wnt2 to promote cancer progression in colorectal cancer. Cancer Med. 2019, 8, 6370–6382. [Google Scholar] [CrossRef] [Green Version]
- Kramer, N.; Schmöllerl, J.; Unger, C.; Nivarthi, H.; Rudisch, A.; Unterleuthner, D.; Scherzer, M.; Riedl, A.; Artaker, M.; Crncec, I.; et al. Autocrine WNT2 signaling in fibroblasts promotes colorectal cancer progression. Oncogene 2017, 36, 5460–5472. [Google Scholar] [CrossRef]
- Unterleuthner, D.; Neuhold, P.; Schwarz, K.; Janker, L.; Neuditschko, B.; Nivarthi, H.; Crncec, I.; Kramer, N.; Unger, C.; Hengstschläger, M.; et al. Cancer-associated fibroblast-derived WNT2 increases tumor angiogenesis in colon cancer. Angiogenesis 2020, 23, 159–177. [Google Scholar] [CrossRef] [Green Version]
- Guraya, S.Y. Prognostic significance of circulating microRNA-21 expression in esophageal, pancreatic and colorectal cancers; a systematic review and meta-analysis. Int. J. Surg. 2018, 60, 41–47. [Google Scholar] [CrossRef]
- Bhome, R.; Goh, R.W.; Bullock, M.D.; Pillar, N.; Thirdborough, S.M.; Mellone, M.; Mirnezami, R.; Galea, D.; Veselkov, K.; Gu, Q.; et al. Exosomal microRNAs derived from colorectal cancer-associated fibroblasts: Role in driving cancer progression. Aging 2017, 9, 2666–2694. [Google Scholar] [CrossRef] [Green Version]
- Bullock, M.D.; Pickard, K.M.; Nielsen, B.S.; Sayan, A.E.; Jenei, V.; Mellone, M.; Mitter, R.; Primrose, J.N.; Thomas, G.J.; Packham, G.K.; et al. Pleiotropic actions of miR-21 highlight the critical role of deregulated stromal microRNAs during colorectal cancer progression. Cell Death Dis. 2013, 4, e684. [Google Scholar] [CrossRef] [Green Version]
- Bu, L.; Baba, H.; Yasuda, T.; Uchihara, T.; Ishimoto, T. Functional diversity of cancer-associated fibroblasts in modulating drug resistance. Cancer Sci. 2020, 111, 3468–3477. [Google Scholar] [CrossRef]
- Goncalves-Ribeiro, S.; Diaz-Maroto, N.G.; Berdiel-Acer, M.; Soriano, A.; Guardiola, J.; Martinez-Villacampa, M.; Salazar, R.; Capella, G.; Villanueva, A.; Martinez-Balibrea, E.; et al. Carcinoma-associated fibroblasts affect sensitivity to oxaliplatin and 5FU in colo-rectal cancer cells. Oncotarget 2016, 7, 59766–59780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Xu, X.; Zhu, J.; Zhang, S.; Wu, Y.; Wu, Y.; Zhao, K.; Xing, C.; Cao, J.; Zhu, H.; et al. miR-31 affects colorectal cancer cells by inhibiting autophagy in cancer-associated fibroblasts. Oncotarget 2016, 7, 79617–79628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Liu, J.; Zhang, Q.; Liu, B.; Cheng, Y.; Zhang, Y.; Sun, Y.; Ge, H.; Liu, Y. Exosome-mediated transfer of miR-93-5p from can-cer-associated fibroblasts confer radioresistance in colorectal cancer cells by downregulating FOXA1 and upregulating TGFB3. J. Exp. Clin. Cancer Res. 2020, 39, 65. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Ding, L.; Zhang, D.; Shi, G.; Xu, Q.; Shen, S.; Wang, Y.; Wang, T.; Hou, Y. Carcinoma-associated fibroblasts promote the stem-ness and chemoresistance of colorectal cancer by transferring exosomal lncRNA H19. Theranostics 2018, 8, 3932–3948. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Ruan, H.; Zhang, X.; Xu, X.; Zhu, Y.; Peng, H.; Zhang, X.; Kong, F.; Guan, M. Long noncoding RNA CCAL transferred from fibroblasts by exosomes promotes chemoresistance of colorectal cancer cells. Int. J. Cancer 2020, 146, 1700–1716. [Google Scholar] [CrossRef]
- Tang, Y.A.; Chen, Y.F.; Bao, Y.; Mahara, S.; Yatim, S.; Oguz, G.; Lee, P.L.; Feng, M.; Cai, Y.; Tan, E.Y.; et al. Hypoxic tumor microenviron-ment activates GLI2 via HIF-1alpha and TGF-beta2 to promote chemoresistance in colorectal cancer. Proc. Natl. Acad. Sci. USA 2018, 115, E5990–E5999. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huynh, L.K.; Hipolito, C.J.; Ten Dijke, P. A Perspective on the Development of TGF-beta Inhibitors for Cancer Treatment. Biomolecules 2019, 9, 743. [Google Scholar] [CrossRef] [Green Version]
- Ungefroren, H. Blockade of TGF-beta signaling: A potential target for cancer immunotherapy? Expert Opin. Ther. Targets 2019, 23, 679–693. [Google Scholar] [CrossRef]
- Villalba, M.; Evans, S.R.; Vidal-Vanaclocha, F.; Calvo, A. Role of TGF-beta in metastatic colon cancer: It is finally time for targeted therapy. Cell Tissue Res. 2017, 370, 29–39. [Google Scholar] [CrossRef]
- Hawinkels, L.J.; Ten Dijke, P. Exploring anti-TGF-beta therapies in cancer and fibrosis. Growth Factors 2011, 29, 140–152. [Google Scholar] [CrossRef]
- Tauriello, D.V.F.; Palomo-Ponce, S.; Stork, D.; Berenguer-Llergo, A.; Badia-Ramentol, J.; Iglesias, M.; Sevillano, M.; Ibiza, S.; Canellas, A.; Hernando-Momblona, X.; et al. TGFbeta drives immune evasion in genetically reconstituted colon cancer metastasis. Nature 2018, 554, 538–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrett, R.; Puré, E. Cancer-associated fibroblasts: Key determinants of tumor immunity and immunotherapy. Curr. Opin. Immunol. 2020, 64, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Harryvan, T.J.; Verdegaal, E.M.E.; Hardwick, J.C.H.; Hawinkels, L.; van der Burg, S.H. Targeting of the Cancer-Associated Fibro-blast-T-Cell Axis in Solid Malignancies. J. Clin. Med. 2019, 8, 1989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mhaidly, R.; Mechta-Grigoriou, F. Fibroblast heterogeneity in tumor micro-environment: Role in immunosuppression and new therapies. Semin. Immunol. 2020, 48, 101417. [Google Scholar] [CrossRef]
- Monteran, L.; Erez, N. The Dark Side of Fibroblasts: Cancer-Associated Fibroblasts as Mediators of Immunosuppression in the Tumor Microenvironment. Front. Immunol. 2019, 10, 1835. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Zhou, J.; Zhang, J.; Li, S.; Wang, H.; Du, J. Cancer-associated fibroblasts promote PD-L1 expression in mice cancer cells via secreting CXCL5. Int. J. Cancer 2019, 145, 1946–1957. [Google Scholar] [CrossRef] [Green Version]
- Payandeh, Z.; Khalili, S.; Somi, M.H.; Mard-Soltani, M.; Baghbanzadeh, A.; Hajiasgharzadeh, K.; Samadi, N.; Baradaran, B. PD-1/PD-L1-dependent immune response in colorectal cancer. J. Cell. Physiol. 2020, 235, 5461–5475. [Google Scholar] [CrossRef]
- Jacobs, J.; Deschoolmeester, V.; Zwaenepoel, K.; Flieswasser, T.; Deben, C.; Bossche, J.V.D.; Hermans, C.; Rolfo, C.; Peeters, M.; De Wever, O.; et al. Unveiling a CD70-positive subset of cancer-associated fibroblasts marked by pro-migratory activity and thriving regulatory T cell accumulation. OncoImmunology 2018, 7, e1440167. [Google Scholar] [CrossRef] [Green Version]
- Schmetterer, K.G.; Neunkirchner, A.; Pickl, W.F. Naturally occurring regulatory T cells: Markers, mechanisms, and manipulation. FASEB J. 2012, 26, 2253–2276. [Google Scholar] [CrossRef]
- Yu, M.; Guo, G.; Huang, L.; Deng, L.; Chang, C.S.; Achyut, B.R.; Canning, M.; Xu, N.; Arbab, A.S.; Bollag, R.J.; et al. CD73 on can-cer-associated fibroblasts enhanced by the A2B-mediated feedforward circuit enforces an immune checkpoint. Nat. Commun. 2020, 11, 515. [Google Scholar] [CrossRef]
- Inoue, S.; Ito, H.; Tsunoda, T.; Murakami, H.; Ebi, M.; Ogasawara, N.; Kasugai, K.; Kasai, K.; Ikeda, H.; Inaguma, S. CD70 expression in tumor-associated fibroblasts predicts worse survival in colorectal cancer patients. Virchows Arch. 2019, 475, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Zhu, Y.; Lance, P. TNF α-activated stromal COX -2 signalling promotes proliferative and invasive potential of colon cancer epithelial cells. Cell Prolif. 2013, 46, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhu, M.; Lance, P. Stromal COX-2 signaling activated by deoxycholic acid mediates proliferation and invasiveness of colorectal epithelial cancer cells. Biochem. Biophys. Res. Commun. 2012, 425, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Francí, C.; Gallén, M.; Alameda, F.; Baró, T.; Iglesias, M.; Virtanen, I.; De Herreros, A.G. Snail1 Protein in the Stroma as a New Putative Prognosis Marker for Colon Tumours. PLoS ONE 2009, 4, e5595. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.F.; Zhang, X.H.; Gu, C.S.; Zhong, Y.; Long, T.; Ma, Y.D.; Hu, Z.Y.; Li, Z.G.; Wang, X.Y. Cancer-associated fibroblasts promote colo-rectal cancer progression by secreting CLEC3B. Cancer Biol. Ther. 2019, 20, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Drev, D.; Harpain, F.; Beer, A.; Stift, A.; Gruber, E.S.; Klimpfinger, M.; Thalhammer, S.; Reti, A.; Kenner, L.; Bergmann, M.; et al. Impact of Fibroblast-Derived SPARC on Invasiveness of Colorectal Cancer Cells. Cancers 2019, 11, 1421. [Google Scholar] [CrossRef] [Green Version]
- Unger, C.; Kramer, N.; Unterleuthner, D.; Scherzer, M.; Burian, A.; Rudisch, A.; Stadler, M.; Schlederer, M.; Lenhardt, D.; Riedl, A.; et al. Stromal-derived IGF2 promotes colon cancer progression via paracrine and autocrine mechanisms. Oncogene 2017, 36, 5341–5355. [Google Scholar] [CrossRef]
- Yang, T.; Zhiheng, H.; Zhanhuai, W.; Qian, X.; Yue, L.; Xiaoxu, G.; Jingsun, W.; Shu, Z.; Ding, K. Increased RAB31 Expression in Cancer-Associated Fibroblasts Promotes Colon Cancer Progression through HGF-MET Signaling. Front. Oncol. 2020, 10, 1747. [Google Scholar] [CrossRef]
- Song, J.; Chen, W.; Cui, X.; Huang, Z.; Wen, D.; Yang, Y.; Yu, W.; Cui, L.; Liu, C.Y. CCBE1 promotes tumor lymphangiogenesis and is negatively regulated by TGFbeta signaling in colorectal cancer. Theranostics 2020, 10, 2327–2341. [Google Scholar]
- Paauwe, M.; Schoonderwoerd, M.J.A.; Helderman, R.; Harryvan, T.J.; Groenewoud, A.; van Pelt, G.W.; Bor, R.; Hemmer, D.M.; Versteeg, H.H.; Snaar-Jagalska, B.E.; et al. Endoglin Expression on Cancer-Associated Fibroblasts Regulates Invasion and Stimulates Col-orectal Cancer Metastasis. Clin. Cancer Res. 2018, 24, 6331–6344. [Google Scholar] [CrossRef] [Green Version]
- Ouahoud, S.; Voorneveld, P.W.; van der Burg, L.R.A.; de Jonge-Muller, E.S.M.; Schoonderwoerd, M.J.A.; Paauwe, M.; de Vos, T.; de Wit, S.; van Pelt, G.W.; Mesker, W.E.; et al. Bidirectional tumor/stroma crosstalk promotes metastasis in mesenchymal colorectal cancer. Oncogene 2020, 39, 2453–2466. [Google Scholar] [CrossRef] [PubMed]
- Peña, C.; Céspedes, M.V.; Lindh, M.B.; Kiflemariam, S.; Mezheyeuski, A.; Edqvist, P.-H.; Hägglöf, C.; Birgisson, H.; Bojmar, L.; Jirström, K.; et al. STC1 Expression By Cancer-Associated Fibroblasts Drives Metastasis of Colorectal Cancer. Cancer Res. 2013, 73, 1287–1297. [Google Scholar] [CrossRef] [Green Version]
- Dekker, E.; Tanis, P.J.; Vleugels, J.L.A.; Kasi, P.M.; Wallace, M.B. Colorectal cancer. Lancet 2019, 394, 1467–1480. [Google Scholar] [CrossRef]
- Tommelein, J.; Verset, L.; Boterberg, T.; Demetter, P.; Bracke, M.; De Wever, O. Cancer-associated fibroblasts connect metasta-sis-promoting communication in colorectal cancer. Front. Oncol. 2015, 5, 63. [Google Scholar] [PubMed] [Green Version]
- Hawinkels, L.J.; Paauwe, M.; Verspaget, H.W.; Wiercinska, E.; van der Zon, J.M.; van der Ploeg, K.; Koelink, P.J.; Lindeman, J.H.; Mesker, W.; ten Dijke, P.; et al. Interaction with colon cancer cells hyperactivates TGF-beta signaling in cancer-associated fibroblasts. Oncogene 2014, 33, 97–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillen Diaz-Maroto, N.; Sanz-Pamplona, R.; Berdiel-Acer, M.; Cimas, F.J.; Garcia, E.; Goncalves-Ribeiro, S.; Albert, N.; Garcia-Vicien, G.; Capella, G.; Moreno, V.; et al. Noncanonical TGFbeta Pathway Relieves the Blockade of IL1beta/TGFbeta-Mediated Crosstalk between Tumor and Stroma: TGFBR1 and TAK1 Inhibition in Colorectal Cancer. Clin. Cancer Res. 2019, 25, 4466–4479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, A.; Greening, D.W.; Xu, R.; Suwakulsiri, W.; Simpson, R.J. Exosomes Derived from the Human Primary Colorectal Cancer Cell Line SW480 Orchestrate Fibroblast-Led Cancer Invasion. Proteomics 2020, 20, e2000016. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, S.; Lai, Q.; Fang, Y.; Wu, C.; Liu, Y.; Li, Q.; Wang, X.; Gu, C.; Chen, J.; et al. Cancer-associated fibroblasts-derived exo-somal miR-17-5p promotes colorectal cancer aggressive phenotype by initiating a RUNX3/MYC/TGF-beta1 positive feedback loop. Cancer Lett. 2020, 491, 22–35. [Google Scholar] [CrossRef]
- Ji, Q.; Zhou, L.; Sui, H.; Yang, L.; Wu, X.; Song, Q.; Jia, R.; Li, R.; Sun, J.; Wang, Z.; et al. Primary tumors release ITGBL1-rich extracellular vesicles to promote distal metastatic tumor growth through fibroblast-niche formation. Nat. Commun. 2020, 11, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Mueller, L.; Goumas, F.A.; Affeldt, M.; Sandtner, S.; Gehling, U.M.; Brilloff, S.; Walter, J.; Karnatz, N.; Lamszus, K.; Rogiers, X.; et al. Stromal fibroblasts in colorectal liver metastases originate from resident fibroblasts and generate an inflammatory microenvi-ronment. Am. J. Pathol. 2007, 171, 1608–1618. [Google Scholar] [CrossRef] [Green Version]
- Mueller, L.; Von Seggern, L.; Schumacher, J.; Goumas, F.; Wilms, C.; Braun, F.; Broering, D.C. TNF-α similarly induces IL-6 and MCP-1 in fibroblasts from colorectal liver metastases and normal liver fibroblasts. Biochem. Biophys. Res. Commun. 2010, 397, 586–591. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Zubeldia, I.; Dotor, J.; Redrado, M.; Bleau, A.M.; Manrique, I.; de Aberasturi, A.L.; Villalba, M.; Calvo, A. Co-migration of colon cancer cells and CAFs induced by TGFbeta(1) enhances liver metastasis. Cell Tissue Res. 2015, 359, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Xiao, N.; Wang, J.; Wang, Z.; Zheng, S.; Shan, S.; Wang, J.; Du, J.; Wang, J. SMC1A recruits tumor-associated-fibroblasts (TAFs) and promotes colorectal cancer metastasis. Cancer Lett. 2017, 385, 39–45. [Google Scholar] [CrossRef]
- Herrera, M.; Galindo-Pumariño, C.; García-Barberán, V.; Peña, C. A Snapshot of the Tumor Microenvironment in Colorectal Cancer: The Liquid Biopsy. Int. J. Mol. Sci. 2019, 20, 6016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ao, Z.; Shah, S.H.; Machlin, L.M.; Parajuli, R.; Miller, P.C.; Rawal, S.; Williams, A.J.; Cote, R.J.; Lippman, M.E.; Datar, R.H.; et al. Identifica-tion of Cancer-Associated Fibroblasts in Circulating Blood from Patients with Metastatic Breast Cancer. Cancer Res. 2015, 75, 4681–4687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, M.L.; Siddiqui, J.; Pienta, K.J.; Getzenberg, R.H. Circulating fibroblast-like cells in men with metastatic prostate cancer. Prostate 2013, 73, 176–181. [Google Scholar] [CrossRef] [Green Version]



| CAF Metadata | Role in CRC Progression | Clinical Relevance | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Name of Factor | Characteristics of CAFs Expressing or Secreting This Factor | Characteristics of Tumors Which the CAFs Originated From | CRC Proliferation | CRC Invasion/Migration | Angiogenesis | Therapy Resistance | Immune Evasion | Organ Metastasis | Association High Expression Levels in CAFs & Poor Clinical Outcomes |
| Exosomal circular RNA SLC7A6 [112] | - | Primary tumor; no neo-adjuvant treatment | ↑ | ↑ | - | - | - | - | Yes [112] |
| HIC-5 [109] | Co-localization with α-SMA | Primary tumor | ↑ | - | - | - | - | - | - |
| COX-2 [142,143] | - | Primary tumor | ↑ | - | - | - | - | - | - |
| Snail-1 [110] | Co-localization with α-SMA and FAP | Primary tumor | ↑ | ↑ | - | - | - | - | Yes [144] |
| FGF-1 [111] | Vimentin-positive | Primary tumor | ↑ | ↑ | ↑ | - | - | - | - |
| FGF-3 [111] | Vimentin-positive | Primary tumor | ↑ | ↑ | ↑ | - | - | - | - |
| Wnt2 [114,115,116] | - | Primary tumor; rectum; stage II-IV; well/moderate differentiation [114]. Not described in [115,116] | ↑ | ↑ | ↑ | - | - | - | Yes [114,115] |
| miRNA-21 [118,119] | - | Primary tumor; rectum & sigmoid; stage II-III; well/moderate differentiation; microsatellite stable; no neo-adjuvant treatment | ↑ | ↑ | - | - | - | ↑ | Yes [117] |
| CD10 [81] | - | Primary tumor; rectum and colon; stage II-III; well/moderate differentiation | ↑ | ↑ | - | - | - | - | - |
| CLEC3B [145] | - | Primary tumor | - | ↑ | - | - | - | - | Yes [145] 1 |
| Podoplanin [79] | - | - | - | ↓ | - | - | - | - | No [79,80] 2 |
| SPARC [146] | - | Primary tumor | - | ↑ | - | - | - | - | Yes [146] |
| IGF-2 [147] | - | Primary tumor | = | ↑ | - | - | - | ↑ | Yes [147] |
| RAB31 [148] | Co-localization with α-SMA and vimentin | Primary tumor | = | ↑ | - | - | - | - | Yes [148] |
| CCBE1 [149] | Co-localization with α-SMA | Primary tumor | - | - | ↑ | - | - | - | Yes [149] |
| miRNA-31 [122] | - | Primary tumor; no neo-adjuvant treatment | - | - | - | ↑ | - | - | - |
| miRNA-93–5p [123] | - | Primary tumor | - | - | - | ↑ | - | - | - |
| CRC-associated lncRNA [125] | FAP-positive, co-localization with α-SMA | Primary tumor | - | - | - | ↑ | - | - | - |
| lncRNA H19 [124] | - | Primary tumor | - | - | - | ↑ | - | - | - |
| TGF-β2 [126] | - | Primary tumor | - | - | - | ↑ | - | - | Yes [126] |
| CXCL5 [136] | - | Primary tumor | - | - | - | - | ↑ | - | - |
| CD70 [138] | Co-localization with α-SMA and FAP | Primary tumor | - | ↑ | - | - | ↑ | - | Yes [138,141] |
| CD73 [140] | Co-localization with α-SMA | Primary tumor | - | - | - | - | ↑ | - | Yes [140] |
| Endoglin [150] | Co-localization with α-SMA | Primary tumor | - | - | - | - | - | ↑ | Yes [150] |
| BMP2 [151] | - | Primary tumor | - | ↑ | - | - | - | ↑ | Yes [151] 3 |
| IL-11 [63] | FAP-positive | Primary tumor | - | - | - | - | - | ↑ | - |
| STC1 [152] | - | Primary tumor | - | ↑ | - | - | - | ↑ | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dang, H.; Harryvan, T.J.; Hawinkels, L.J.A.C. Fibroblast Subsets in Intestinal Homeostasis, Carcinogenesis, Tumor Progression, and Metastasis. Cancers 2021, 13, 183. https://doi.org/10.3390/cancers13020183
Dang H, Harryvan TJ, Hawinkels LJAC. Fibroblast Subsets in Intestinal Homeostasis, Carcinogenesis, Tumor Progression, and Metastasis. Cancers. 2021; 13(2):183. https://doi.org/10.3390/cancers13020183
Chicago/Turabian StyleDang, Hao, Tom J. Harryvan, and Lukas J. A. C. Hawinkels. 2021. "Fibroblast Subsets in Intestinal Homeostasis, Carcinogenesis, Tumor Progression, and Metastasis" Cancers 13, no. 2: 183. https://doi.org/10.3390/cancers13020183