Development of Discordant Hypermetabolic Prostate Cancer Lesions in the Course of [177Lu]PSMA Radioligand Therapy and Their Possible Influence on Patient Outcome
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Cohort
2.2. Imaging and Treatment Protocol
2.3. Image Analysis
2.4. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
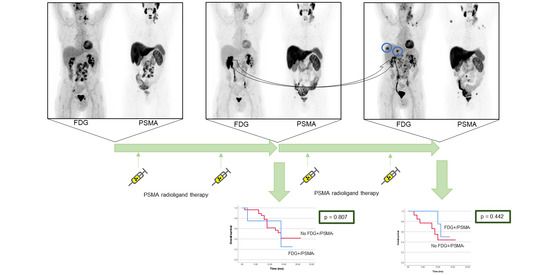
3.2. Dual Tracer Staging after Two Cycles of PSMA RLT
3.3. Dual Tracer Staging after Four Cycles of PSMA RLT
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statements
Conflicts of Interest
References
- Ryan, C.J.; Smith, M.R.; De Bono, J.S.; Molina, A.; Logothetis, C.J.; De Souza, P.; Fizazi, K.; Mainwaring, P.; Piulats, J.M.; Vogelzang, N.J.; et al. Abiraterone in Metastatic Prostate Cancer without Previous Chemotherapy. N. Engl. J. Med. 2013, 368, 138–148. [Google Scholar] [CrossRef] [Green Version]
- Beer, T.M.; Armstrong, A.J.; Rathkopf, D.E.; Loriot, Y.; Sternberg, C.N.; Higano, C.S.; Iversen, P.; Bhattacharya, S.; Carles, J.; Chowdhury, S.; et al. Enzalutamide in Metastatic Prostate Cancer before Chemotherapy. N. Engl. J. Med. 2014, 371, 424–433. [Google Scholar] [CrossRef] [Green Version]
- Tannock, I.F.; De Wit, R.; Berry, W.R.; Horti, J.; Pluzanska, A.; Chi, K.N.; Oudard, S.; Theodore, C.; James, N.D.; Turesson, I.; et al. Docetaxel plus Prednisone or Mitoxantrone plus Prednisone for Advanced Prostate Cancer. N. Engl. J. Med. 2004, 351, 1502–1512. [Google Scholar] [CrossRef] [Green Version]
- Berthold, D.R.; Pond, G.R.; Soban, F.; De Wit, R.; Eisenberger, M.; Tannock, I.F. Docetaxel Plus Prednisone or Mitoxantrone Plus Prednisone for Advanced Prostate Cancer: Updated Survival in the TAX 327 Study. J. Clin. Oncol. 2008, 26, 242–245. [Google Scholar] [CrossRef] [PubMed]
- De Bono, J.S.; Oudard, S.; Özgüroglu, M.; Hansen, S.; Machiels, J.-P.; Kocak, I.; Gravis, G.; Bodrogi, I.; Mackenzie, M.J.; Shen, L.; et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet 2010, 376, 1147–1154. [Google Scholar] [CrossRef]
- Kim, Y.J. Therapeutic Responses and Survival Effects of 177Lu-PSMA-617 Radioligand Therapy in Metastatic Castrate-Resistant Prostate Cancer. Clin. Nucl. Med. 2018, 43, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, K.; Bodei, L.; Morris, M.J. Is the Vision of Radioligand Therapy for Prostate Cancer Becoming a Reality? An Overview of the Phase III VISION Trial and Its Importance for the Future of Theranostics. J. Nucl. Med. 2019, 60, 1504–1506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sartor, O.; de Bono, J.; Chi, K.N.; Fizazi, K.; Herrmann, K.; Rahbar, K.; Tagawa, S.T.; Nordquist, L.T.; Vaishampayan, N.; El-Haddad, G.; et al. Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N. Engl. J. Med. 2021. [Google Scholar] [CrossRef]
- Kratochwil, C.; Fendler, W.P.; Eiber, M.; Baum, R.; Bozkurt, M.F.; Czernin, J.; Bolton, R.C.D.; Ezziddin, S.; Forrer, F.; Hicks, R.J.; et al. EANM procedure guidelines for radionuclide therapy with 177Lu-labelled PSMA-ligands (177Lu-PSMA-RLT). Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 2536–2544. [Google Scholar] [CrossRef]
- Jadvar, H. Imaging evaluation of prostate cancer with 18F-fluorodeoxyglucose PET/CT: Utility and limitations. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 5–10. [Google Scholar] [CrossRef]
- Jadvar, H. Is There Use for FDG-PET in Prostate Cancer? Semin. Nucl. Med. 2016, 46, 502–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jadvar, H.; Desai, B.; Ji, L.; Conti, P.S.; Dorff, T.B.; Groshen, S.G.; Pinski, J.K.; Quinn, D. Baseline 18F-FDG PET/CT Parameters as Imaging Biomarkers of Overall Survival in Castrate-Resistant Metastatic Prostate Cancer. J. Nucl. Med. 2013, 54, 1195–1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suman, S.; Parghane, R.V.; Joshi, A.; Prabhash, K.; Bakshi, G.; Talole, S.; Banerjee, S.; Basu, S. Therapeutic efficacy, prognostic variables and clinical outcome of 177Lu-PSMA-617 PRLT in progressive mCRPC following multiple lines of treatment: Prognostic implications of high FDG uptake on dual tracer PET-CT vis-à-vis Gleason score in such cohort. Br. J. Radiol. 2019, 92, 20190380. [Google Scholar] [CrossRef]
- Hofman, M.S.; Violet, J.; Hicks, R.J.; Ferdinandus, J.; Thang, S.P.; Akhurst, T.; Iravani, A.; Kong, G.; Kumar, A.R.; Murphy, D.G.; et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): A single-centre, single-arm, phase 2 study. Lancet Oncol. 2018, 19, 825–833. [Google Scholar] [CrossRef]
- Violet, J.; Sandhu, S.; Iravani, A.; Ferdinandus, J.; Thang, S.P.; Kong, G.; Ravi Kumar, A.; Akhurst, T.; Pattison, D.A.; Beaulieu, A.; et al. Long term follow-up and outcomes of re-treatment in an expanded 50 patient single-center phase II prospective trial of Lutetium-177 ((177)Lu) PSMA-617 theranostics in metastatic castrate-resistant prostate cancer. J. Nucl. Med. 2019, 15, 236414. [Google Scholar]
- Thang, S.P.; Violet, J.; Sandhu, S.; Iravani, A.; Akhurst, T.; Kong, G.; Kumar, A.R.; Murphy, D.G.; Williams, S.G.; Hicks, R.J.; et al. Poor Outcomes for Patients with Metastatic Castration-resistant Prostate Cancer with Low Prostate-specific Membrane Antigen (PSMA) Expression Deemed Ineligible for 177Lu-labelled PSMA Radioligand Therapy. Eur. Urol. Oncol. 2019, 2, 670–676. [Google Scholar] [CrossRef]
- Michalski, K.; Ruf, J.; Goetz, C.; Seitz, A.K.; Buck, A.K.; Lapa, C.; Hartrampf, P.E. Prognostic implications of dual tracer PET/CT: PSMA ligand and [18F]FDG PET/CT in patients undergoing [177Lu]PSMA radioligand therapy. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 2024–2030. [Google Scholar] [CrossRef]
- Alipour, R.; Azad, A.; Hofman, M.S. Guiding management of therapy in prostate cancer: Time to switch from conventional imaging to PSMA PET? Ther. Adv. Med. Oncol. 2019, 11, 1758835919876828. [Google Scholar] [CrossRef]
- Eiber, M.; Herrmann, K.; Calais, J.; Hadaschik, B.; Giesel, F.L.; Hartenbach, M.; Hope, T.A.; Reiter, R.; Maurer, T.; Weber, W.A.; et al. Prostate Cancer Molecular Imaging Standardized Evaluation (PROMISE): Proposed miTNM Classification for the Interpretation of PSMA-Ligand PET/CT. J. Nucl. Med. 2018, 59, 469–478. [Google Scholar] [CrossRef] [Green Version]
- Rosar, F.; Ribbat, K.; Ries, M.; Linxweiler, J.; Bartholomä, M.; Maus, S.; Schreckenberger, M.; Ezziddin, S.; Khreish, F. Neuron-specific enolase has potential value as a biomarker for [18F]FDG/[68Ga]Ga-PSMA-11 PET mismatch findings in advanced mCRPC patients. EJNMMI Res. 2020, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Emmett, L.; Crumbaker, M.; Ho, B.; Willowson, K.; Eu, P.; Ratnayake, L.; Epstein, R.; Blanksby, A.; Horvath, L.; Guminski, A.; et al. Results of a Prospective Phase 2 Pilot Trial of 177Lu–PSMA-617 Therapy for Metastatic Castration-Resistant Prostate Cancer Including Imaging Predictors of Treatment Response and Patterns of Progression. Clin. Genitourin. Cancer 2019, 17, 15–22. [Google Scholar] [CrossRef]
- Hartrampf, P.E.; Seitz, A.K.; Krebs, M.; Buck, A.K.; Lapa, C. False-negative 18F-PSMA-1007 PET/CT in metastatic prostate cancer related to high physiologic liver uptake. Eur. J. Nucl. Med. Mol. Imaging 2019, 47, 2044–2046. [Google Scholar] [CrossRef] [PubMed]
- Kessel, K.; Seifert, R.; Schäfers, M.; Weckesser, M.; Schlack, K.; Boegemann, M.; Rahbar, K. Second line chemotherapy and visceral metastases are associated with poor survival in patients with mCRPC receiving 177Lu-PSMA-617. Theranostics 2019, 9, 4841–4848. [Google Scholar] [CrossRef] [PubMed]
- Heck, M.M.; Tauber, R.; Schwaiger, S.; Retz, M.; D’Alessandria, C.; Maurer, T.; Gafita, A.; Wester, H.-J.; Gschwend, J.E.; Weber, W.A.; et al. Treatment Outcome, Toxicity, and Predictive Factors for Radioligand Therapy with 177Lu-PSMA-I&T in Metastatic Castration-resistant Prostate Cancer. Eur. Urol. 2019, 75, 920–926. [Google Scholar]
- Khreish, F.; Kochems, N.; Rosar, F.; Sabet, A.; Ries, M.; Maus, S.; Saar, M.; Bartholomä, M.; Ezziddin, S. Response and outcome of liver metastases in patients with metastatic castration-resistant prostate cancer (mCRPC) undergoing 177Lu-PSMA-617 radioligand therapy. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Ceci, F.; Bianchi, L.; Borghesi, M.; Polverari, G.; Farolfi, A.; Briganti, A.; Schiavina, R.; Brunocilla, E.; Castellucci, P.; Fanti, S. Prediction nomogram for 68Ga-PSMA-11 PET/CT in different clinical settings of PSA failure after radical treatment for prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2019, 47, 136–146. [Google Scholar] [CrossRef]
- Bianchi, L.; Borghesi, M.; Schiavina, R.; Castellucci, P.; Ercolino, A.; Bianchi, F.M.; Barbaresi, U.; Polverari, G.; Brunocilla, E.; Fanti, S.; et al. Predictive accuracy and clinical benefit of a nomogram aimed to predict 68Ga-PSMA PET/CT positivity in patients with prostate cancer recurrence and PSA < 1 ng/ml external validation on a single institution database. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2100–2105. [Google Scholar]




| After 2 Cycles of PSMA RLT (n = 32) | After 4 Cycles of PSMA RLT (n = 18) | |||
|---|---|---|---|---|
| Mean ± SD (Range) | Mean ± SD (Range) | |||
| Age (years) | 72.6 ± 8.9 (46–90) | 75.3 ± 9.8 (46–85) | ||
| Time since diagnosis of prostate cancer (years) | 8.1 ± 6.6 (2–26) | 10.1 ± 7.8 (2–26) | ||
| Gleason score at diagnosis | (7–10) * | (7–9) ** | ||
| ECOG before PSMA RLT | (0–2) | (0–2) | ||
| PSA before PSMA RLT [ng/mL] | 389 ± 709 (5–2650) | 384 ± 670 (5–2650) | ||
| Sites of disease before PSMA RLT | n | % | n | % |
| Prostate/local | 13 | 41 | 7 | 39 |
| Lymph node | 17 | 53 | 9 | 50 |
| Bone | 30 | 94 | 18 | 100 |
| Liver | 3 | 9 | 1 | 6 |
| Lung | 4 | 13 | 3 | 17 |
| Other | 3 | 9 | 2 | 11 |
| Previous treatment | n | % | n | % |
| Prostatectomy | 17 | 53 | 11 | 61 |
| Radiotherapy to prostate/prostate bed | 15 | 47 | 9 | 50 |
| ADT | 32 | 100 | 18 | 100 |
| Abiraterone | 25 | 78 | 12 | 67 |
| Enzalutamide | 19 | 59 | 13 | 72 |
| Docetaxel | 21 | 66 | 11 | 61 |
| Cabazitaxel | 7 | 22 | 4 | 22 |
| Other | 6 | 19 | 3 | 11 |
| Pat. # | Before PSMA RLT | After 2 Cycles of PSMA RLT | After 4 Cycles of PSMA RLT | ||||
|---|---|---|---|---|---|---|---|
| # of Metastases on PSMA PET/CT | # of Metastases on PSMA PET/CT | # of Discordant Lesions | Discordant Organs | # of Metastases on PSMA PET/CT | # of Discordant Lesions | Discordant Organs | |
| 3 | >10 | DBMI | 1 | OSS | − | − | − |
| 4 | DBMI | DBMI | 2 | HEP * | − | − | − |
| 7 | >10 | >10 | n.a. | n.a. | >10 | 1 | OSS |
| 10 | >3 but <10 | >3 but <10 | 4 | HEP **, PeC | >10 | 7 | HEP **, PeC |
| 14 | >10 | >10 | n.a. | n.a. | >10 | 3 | HEP |
| 23 | >10 | >10 | 1 | LN | >10 | 9 | LN |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hartrampf, P.E.; Lapa, C.; Serfling, S.E.; Buck, A.K.; Seitz, A.K.; Meyer, P.T.; Ruf, J.; Michalski, K. Development of Discordant Hypermetabolic Prostate Cancer Lesions in the Course of [177Lu]PSMA Radioligand Therapy and Their Possible Influence on Patient Outcome. Cancers 2021, 13, 4270. https://doi.org/10.3390/cancers13174270
Hartrampf PE, Lapa C, Serfling SE, Buck AK, Seitz AK, Meyer PT, Ruf J, Michalski K. Development of Discordant Hypermetabolic Prostate Cancer Lesions in the Course of [177Lu]PSMA Radioligand Therapy and Their Possible Influence on Patient Outcome. Cancers. 2021; 13(17):4270. https://doi.org/10.3390/cancers13174270
Chicago/Turabian StyleHartrampf, Philipp E., Constantin Lapa, Sebastian E. Serfling, Andreas K. Buck, Anna Katharina Seitz, Philipp T. Meyer, Juri Ruf, and Kerstin Michalski. 2021. "Development of Discordant Hypermetabolic Prostate Cancer Lesions in the Course of [177Lu]PSMA Radioligand Therapy and Their Possible Influence on Patient Outcome" Cancers 13, no. 17: 4270. https://doi.org/10.3390/cancers13174270