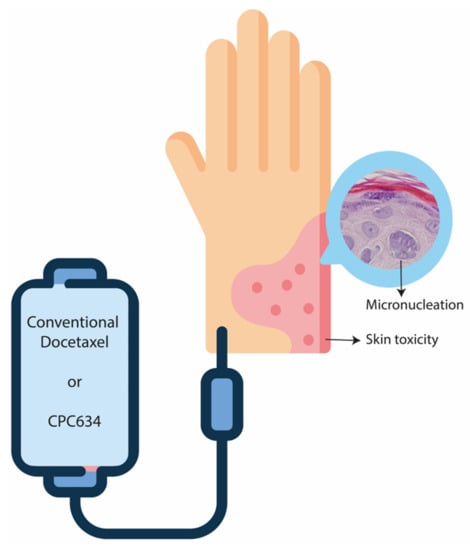
Docetaxel Skin Exposure and Micronucleation Contributes to Skin Toxicity Caused by CPC634
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Skin Biopsies
2.3. Pharmacokinetic Analysis
2.4. Histopathological Analysis
2.5. Statistics
3. Results
3.1. Pharmacokinetics
3.2. Histopathological Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Herbst, R.S.; Khuri, F.R. Mode of action of docetaxel—A basis for combination with novel anticancer agents. Cancer Treat. Rev. 2003, 29, 407–415. [Google Scholar] [CrossRef]
- Engels, F.K.; Verweij, J. Docetaxel administration schedule: From fever to tears? A review of randomised studies. Eur. J. Cancer 2005, 41, 1117–1126. [Google Scholar] [CrossRef] [PubMed]
- Ho, M.; Mackey, J. Presentation and management of docetaxel-related adverse effects in patients with breast cancer. Cancer Manag. Res. 2014, 6, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Siles, M.; Arenas-Villafranca, J.J.; Pérez-Ruiz, E.; Fernández, M.F.D.L.; Tortajada, B.; Rivas-Ruiz, F.; Faus, V.; Rueda, A.; Goitia, B.T. New cutaneous toxicities with generic docetaxel: Are the excipients guilty? Support. Care Cancer 2015, 23, 1917–1923. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K.; Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Eerden, R.A.G.; Mathijssen, R.H.J.; Koolen, S.L.W. Recent Clinical Developments of Nanomediated Drug Delivery Systems of Taxanes for the Treatment of Cancer. Int. J. Nanomed. 2020, 15, 8151–8166. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Maeda, H. A new concept for macromolecular therapeutics in cancer chemotherapy: Mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986, 46, 6387–6392. [Google Scholar] [PubMed]
- Talelli, M.; Barz, M.; Rijcken, C.J.; Kiessling, F.; Hennink, W.E.; Lammers, T. Core-crosslinked polymeric micelles: Principles, preparation, biomedical applications and clinical translation. Nano Today 2015, 10, 93–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Q.; Rijcken, C.J.; Bansal, R.; Hennink, W.E.; Storm, G.; Prakash, J. Complete regression of breast tumour with a single dose of docetaxel-entrapped core-cross-linked polymeric micelles. Biomaterials 2015, 53, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Atrafi, F.; van Eerden, R.A.G.; van Hylckama Vlieg, M.A.M.; Oomen-de Hoop, E.; De Bruijn, P.; Lolkema, M.P.; Moelker, A.; Rijcken, C.J.; Hanssen, R.; Sparreboom, A.; et al. Intratumoral Comparison of Nanoparticle Entrapped Docetaxel (CPC634) with Conventional Docetaxel in Patients with Solid Tumors. Clin. Cancer Res. 2020, 26, 3537–3545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atrafi, F.; Dumez, H.; Mathijssen, R.H.; Menke van der Houven van Oordt, C.W.; Rijcken, C.J.; Hanssen, R.; Eskens, F.A.; Schöffski, P. A phase I dose-escalation and pharmacokinetic study of a micellar nanoparticle with entrapped docetaxel (CPC634) in patients with advanced solid tumours. J. Control. Release 2020, 325, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Braal, C.L.; de Bruijn, P.; Atrafi, F.; van Geijn, M.; Rijcken, C.J.F.; Mathijssen, R.H.J.; Koolen, S.L.W. A new method for the determination of total and released docetaxel from docetaxel-entrapped core-crosslinked polymeric micelles (CriPec(R)) by LC-MS/MS and its clinical application in plasma and tissues in patients with various tumours. J Pharm Biomed Anal. 2018, 161, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.; Kenward, M.G. Design and Analysis of Cross-Over Trials; CRC Press: Boca Raton, PL, USA, 2014. [Google Scholar]
- Poi, M.; Berger, M.; Lustberg, M.; Layman, R.; Shapiro, C.L.; Ramaswamy, B.; Mrozek, E.; Olson, E.; Wesolowski, R. Docetaxel-induced skin toxicities in breast cancer patients subsequent to paclitaxel shortage: A case series and literature review. Support. Care Cancer 2013, 21, 2679–2686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortes, J.E.; Pazdur, R. Docetaxel. J. Clin. Oncol. 1995, 13, 2643–2655. [Google Scholar] [CrossRef] [PubMed]
- Sibaud, V.; Lebœuf, N.R.; Roche, H.; Belum, V.R.; Gladieff, L.; Deslandres, M.; Montastruc, M.; Eche, A.; Vigarios, E.; Dalenc, F.; et al. Dermatological adverse events with taxane chemotherapy. Eur. J. Dermatol. 2016, 26, 427–443. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, C.; Hutton, B.; Mazzarello, S.; Smith, S.; Joy, A.; Amir, E.; Ibrahim, M.F.K.; Gregario, N.; Daigle, K.; Eggert, L.; et al. Optimisation of steroid prophylaxis schedules in breast cancer patients receiving docetaxel chemotherapy—A survey of health care providers and patients. Support. Care Cancer 2015, 23, 3269–3275. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, G.C.; Keeling, J.H.; Burris, H.A.; Cook, G.; Irvin, R.; Kuhn, J.; McCollough, M.L.; Von Hoff, D.D. Acute Cutaneous Reactions to Docetaxel, a New Chemotherapeutic Agent. Arch. Dermatol. 1995, 131, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Greaves, M.W. Anti-inflammatory action of corticosteroids. Postgrad. Med. J. 1976, 52, 631–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchison, T.J.; Pineda, J.; Shi, J.; Florian, S. Is inflammatory micronucleation the key to a successful anti-mitotic cancer drug? Open Biol. 2017, 7, 170182. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Geometric Mean Ratio | 95% Confidence Interval | Significance (2-Tailed) | |
|---|---|---|---|
| Tumour TDx vs. skin TDx | 3.501 | 2.197–5.579 | <0.001 |
| Tumour RDx vs. skin RDx | 2.918 | 1.960–4.345 | <0.001 |
| Tumour Cd vs. skin Cd | 3.133 | 1.745–5.618 | 0.001 |
| Pearson’s Correlation | Significance (2-Tailed) | |
|---|---|---|
| Tumour TDx vs. skin TDx | 0.224 | 0.342 |
| Tumour RDx vs. skin RDx | 0.220 | 0.351 |
| Tumour Cd vs. skin Cd | 0.110 | 0.663 |
| AUCinf TDx vs. skin TDx | 0.351 | 0.129 |
| AUCinf RDx vs. skin RDx | 0.477 | 0.034 |
| AUCinf Cd vs. skin Cd | 0.441 | 0.052 |
| Cmax TDx vs. skin TDx | 0.322 | 0.166 |
| Cmax RDx vs. skin RDx | 0.288 | 0.218 |
| Cmax Cd vs. skin Cd | 0.478 | 0.033 |
| Treatment Arm | Apoptosis | Mitosis | Micronucleation | Atypia | Inflammation | Ki67 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | CPC634 | Cd | Baseline | CPC634 | Cd | Baseline | CPC634 | Cd | Baseline | CPC634 | Cd | Baseline | CPC634 | Cd | Baseline | CPC634 | Cd | |
| A | 0 | 13 | 3 | 0 | 10 | 0 | 0 | 2 | 1 | 0 | 2 | 1 | 1 | 1 | 1 | 1% | 50% | 5% |
| A | 0 | 11 | 3 | 1 | 4 | 4 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 5% | 5% | 5% |
| A | 5 | 97 | 8 | 1 | 31 | 6 | 0 | 3 | 2 | 0 | 1 | 1 | 1 | 1 | 1 | 5% | 50% | 25% |
| A | 1 | 9 | 4 | 0 | 7 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 5% | 5% | 5% |
| A | 0 | 21 | 6 | 0 | 5 | 9 | 0 | 1 | 3 | 0 | 0 | 2 | 1 | 1 | 1 | 5% | 5% | 5% |
| A | 0 | 9 | 26 | 0 | 3 | 8 | 0 | 1 | 2 | 0 | 1 | 1 | 1 | 1 | 1 | 5% | 5% | 50% |
| A | 0 | 7 | NA | 0 | 2 | NA | 0 | 1 | NA | 0 | 1 | NA | 1 | 1 | NA | 5% | 5% | NA |
| B | 0 | 0 | 8 | 0 | 4 | 0 | 0 | 2 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 5% | 5% | 25% |
| B | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 5% | 5% | 5% |
| B | 0 | 12 | 8 | 3 | 3 | 0 | 0 | 2 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 5% | 50% | 5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atrafi, F.; van Eerden, R.A.G.; Koolen, S.L.W.; de Bruijn, P.; Rijcken, C.J.F.; Hanssen, R.; Eskens, F.A.L.M.; Lolkema, M.P.; Oomen-de Hoop, E.; Damman, J.; et al. Docetaxel Skin Exposure and Micronucleation Contributes to Skin Toxicity Caused by CPC634. Cancers 2021, 13, 3741. https://doi.org/10.3390/cancers13153741
Atrafi F, van Eerden RAG, Koolen SLW, de Bruijn P, Rijcken CJF, Hanssen R, Eskens FALM, Lolkema MP, Oomen-de Hoop E, Damman J, et al. Docetaxel Skin Exposure and Micronucleation Contributes to Skin Toxicity Caused by CPC634. Cancers. 2021; 13(15):3741. https://doi.org/10.3390/cancers13153741
Chicago/Turabian StyleAtrafi, Florence, Ruben A. G. van Eerden, Stijn L. W. Koolen, Peter de Bruijn, Cristianne J. F. Rijcken, Rob Hanssen, Ferry A. L. M. Eskens, Martijn P. Lolkema, Esther Oomen-de Hoop, Jeffrey Damman, and et al. 2021. "Docetaxel Skin Exposure and Micronucleation Contributes to Skin Toxicity Caused by CPC634" Cancers 13, no. 15: 3741. https://doi.org/10.3390/cancers13153741