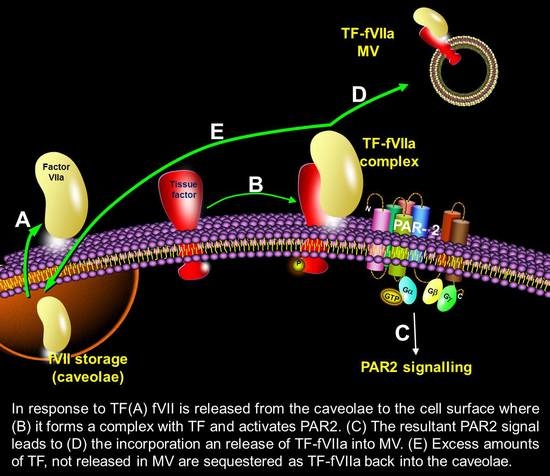
Factor VIIa Regulates the Level of Cell-Surface Tissue Factor through Separate but Cooperative Mechanisms
Abstract
:Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Cell Culture and Analysis of Cell Numbers
2.2. Suppression of fVII/fVIIa Expression by siRNA Transfection
2.3. Duolink Proximity Ligation (PLA) Assay
2.4. Disruption and Labelling of Caveolae by MβCD and NBD-Cholesterol Exchange
2.5. Preparation of Texas Red-Labelled TF
2.6. Statistical Analysis
3. Results
3.1. Assessment of the Interaction of TF, fVII/fVIIa and PAR2 on the Surface of Cells
3.2. In the Presence of fVIIa, Exogenous TF Translocates to the Caveloae on Endothelial Cells
3.3. Lipid Rafts Moderate the Signalling Function of TF-FVIIa on Endothelial Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TF | Tissue factor |
| fVII/X | Factor VII/X |
| fVIIa/Xa | Activated factor VII/Xa |
| HDBEC | Human dermal blood endothelial cells |
| PAR2 | Protease activated receptor 2 |
| MβCD | Methy-β-cyclodextrin |
| MV | Microvesicles |
| TF-KO cells | Tissue factor-knockout cells |
References
- De Blaineville, H.M.D. Injection de matière cerebrale dans des veines. Gaz. Med. Paris (Ser. 2) 1834, 2, 524. [Google Scholar]
- Bächli, E. History of Tissue factor. Br. J. Haematol. 2000, 110, 248–255. [Google Scholar]
- Howell, W.H. The nature and action of the thromboplastin (zymoplastic) substance of the tissues. Am. J. Physiol. 1912, 1, 31–59. [Google Scholar]
- Carson, S.D.; Konigsberg, W.H. Lipid activation of coagulation factor III apoprotein (tissue factor)—Reconstitution of the protein-membrane complex. Thromb. Haemost. 1980, 44, 12–15. [Google Scholar] [CrossRef]
- Kurosawa, S.; Matsuda, M.; Aoki, N. Urinary procoagulant behaves as tissue factor by promoting factor VIIa-catalyzed activation of factor X. Thromb. Res. 1984, 33, 595–606. [Google Scholar] [CrossRef]
- Sanders, N.L.; Bajaj, S.P.; Zivelin, A.; Rapaport, S.I. Inhibition of tissue factor/factor VIIa activity in plasma requires factor X and an additional plasma component. Blood 1985, 66, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Nemerson, Y.; Repke, D. Tissue factor accelerates the activation of coagulation factor VII: The role of a bifunctional coagulation cofactor. Thromb. Res. 1985, 40, 351–358. [Google Scholar] [CrossRef]
- Rao, L.V.; Rapaport, S.I.; Bajaj, S.P. Activation of human factor VII in the initiation of tissue factor-dependent coagulation. Blood 1986, 68, 685–691. [Google Scholar] [CrossRef] [Green Version]
- Rao, L.V.; Pendurthi, U.R. Tissue factor-factor VIIa signaling. Arter. Thromb. Vasc. Biol. 2005, 25, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Rak, J.; Milsom, C.; Yu, J. Tissue factor in cancer. Curr. Opin. Hematol. 2008, 15, 522–528. [Google Scholar] [CrossRef]
- Schaffner, F.; Ruf, W. Tissue factor and PAR2 signaling in the tumor microenvironment. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1999–2004. [Google Scholar] [CrossRef]
- Pradier, A.; Ettelaie, C. The influence of exogenous tissue factor on the regulators of proliferation and apoptosis in endothelial cells. J. Vasc. Res. 2008, 45, 19–32. [Google Scholar] [CrossRef]
- ElKeeb, A.M.; Collier, M.E.; Maraveyas, A.; Ettelaie, C. Accumulation of tissue factor in endothelial cells induces cell apoptosis, mediated through p38 and p53 activation. Thromb. Haemost. 2015, 114, 364–378. [Google Scholar] [PubMed] [Green Version]
- Ethaeb, A.M.; Mohammad, M.A.; Madkhali, Y.; Featherby, S.; Maraveyas, A.; Greenman, J.; Ettelaie, C. Accumulation of tissue factor in endothelial cells promotes cellular apoptosis through over-activation of Src1 and involves β1-integrin signalling. Apoptosis 2020, 25, 29–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madkhali, Y.; Featherby, S.; Collier, M.E.W.; Maraveyas, A.; Greenman, J.; Ettelaie, C. The ratio of factor VIIa:tissue factor content within microvesicles determines the differential influence on endothelial cells. THOpen 2019, 3, e132–e145. [Google Scholar] [CrossRef] [Green Version]
- Featherby, S.; Madkhali, Y.; Maraveyas, A.; Ettelaie, C. Apixaban suppresses the release of TF-positive microvesicles and restrains cancer cell proliferation through directly inhibiting TF-fVIIa activity. Thromb. Haemost. 2019, 119, 1419–1432. [Google Scholar] [CrossRef] [PubMed]
- Riewald, M.; Ruf, W. Mechanistic coupling of protease signaling and initiation of coagulation by tissue factor. Proc. Natl. Acad. Sci. USA 2001, 98, 7742–7747. [Google Scholar] [CrossRef] [Green Version]
- Camerer, E.; Huang, W.; Coughlin, S.R. Tissue factor- and factor X-dependent activation of protease-activated receptor 2 by factor VIIa. Proc. Natl. Acad. Sci. USA 2000, 97, 5255–5260. [Google Scholar] [CrossRef] [Green Version]
- Collier, M.E.; Ettelaie, C. Regulation of the incorporation of tissue factor into microparticles by serine phosphorylation of the cytoplasmic domain of tissue factor. J. Biol. Chem. 2011, 286, 11977–11984. [Google Scholar] [CrossRef] [Green Version]
- Collier, M.E.; Mah, P.M.; Xiao, Y.; Maraveyas, A.; Ettelaie, C. Microparticle-associated tissue factor is recycled by endothelial cells resulting in enhanced surface tissue factor activity. Thromb. Haemost. 2013, 110, 966–976. [Google Scholar]
- Hamik, A.; Setiadi, H.; Bu, G.; McEver, R.P.; Morrisseym, J.H. Down-regulation of monocyte tissue factor mediated by tissue factor pathway inhibitor and the low density lipoprotein receptor-related protein. J. Biol. Chem. 1999, 274, 4962–4969. [Google Scholar] [CrossRef] [Green Version]
- Hansen, C.B.; Pykem, C.; Petersen, L.C.; Rao, L.V. Tissue factor-mediated endocytosis, recycling, and degradation of factor VIIa by a clathrin-independent mechanism not requiring the cytoplasmic domain of tissue factor. Blood 2001, 97, 1712–1720. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, T.; Ohga, N.; Akiyama, K.; Hirata, N.; Kitahara, S.; Maishi, N.; Osawa, T.; Yamamoto, K.; Kondoh, M.; Shindoh, M.; et al. Tumor-derived microvesicles induce proangiogenic phenotype in endothelial cells via endocytosis. PLoS ONE 2012, 7, e34045. [Google Scholar] [CrossRef]
- Mandal, S.K.; Iakhiaev, A.; Pendurthi, U.R.; Rao, L.V. Acute cholesterol depletion impairs functional expression of tissue factor in fibroblasts: Modulation of tissue factor activity by membrane cholesterol. Blood 2005, 105, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Mulder, A.B.; Smit, J.W.; Bom, V.J.; Blom, N.R.; Halie, M.R.; van der Meer, J. Association of endothelial tissue factor and thrombomodulin with caveolae. Blood 1996, 88, 3667–3670. [Google Scholar] [CrossRef] [Green Version]
- Mulder, A.B.; Smit, J.W.; Bom, V.J.; Blom, N.R.; Ruiters, M.H.; Halie, M.R.; van der Meer, J. Association of smooth muscle cell tissue factor with caveolae. Blood 1996, 88, 1306–1313. [Google Scholar] [CrossRef]
- Mandal, S.K.; Pendurthi, U.R.; Rao, L.V. Cellular localization and trafficking of tissue factor. Blood 2006, 107, 4746–4753. [Google Scholar] [CrossRef]
- Awasthi, V.; Mandal, S.K.; Papanna, V.; Rao, L.V.; Pendurthi, U.R. Modulation of tissue factor-factor VIIa signaling by lipid rafts and caveolae. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1447–1455. [Google Scholar] [CrossRef] [Green Version]
- Åberg, M.; Edén, D.; Siegbahn, A. Activation of β1 integrins and caveolin-1 by TF/FVIIa promotes IGF-1R signaling and cell survival. Apoptosis 2020, 25, 519–534. [Google Scholar] [CrossRef]
- Dietzen, D.J.; Page, K.L.; Tetzloff, T.A. Lipid rafts are necessary for tonic inhibition of cellular tissue factor procoagulant activity. Blood 2004, 103, 3038–3044. [Google Scholar] [CrossRef]
- Collier, M.E.W.; Ettelaie, C.; Goult, B.T.; Maraveyas, A.; Goodall, A.H. Investigation of the Filamin A-Dependent Mechanisms of Tissue Factor Incorporation into Microvesicles. Thromb. Haemost. 2017, 117, 2034–2044. [Google Scholar] [CrossRef] [Green Version]
- Mohammad, M.A.; Greenman, J.; Maraveyas, A.; Ettelaie, C. Activation of PAR2 by tissue factor induces the release of the PTEN from MAGI proteins and regulates PTEN and Akt activities. Sci. Rep. 2020, 10, 20908. [Google Scholar] [CrossRef] [PubMed]
- Rondon, A.M.R.; de Almeida, V.H.; Gomes, T.; Verçoza, B.R.F.; Carvalho, R.S.; König, S.; Rodrigues, J.C.F.; Mermelstein, C.D.S.; Versteeg, H.H.; Monteiro, R.Q. Tissue factor mediates microvesicles shedding from MDA-MB-231 breast cancer cells. Biochem. Biophys. Res. Commun. 2018, 502, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Ettelaie, C.; Collier, M.E.; Mei, M.P.; Xiao, Y.P.; Maraveyas, A. Enhanced binding of tissue factor-microparticles to collagen-IV and fibronectin leads to increased tissue factor activity in vitro. Thromb. Haemost. 2013, 109, 61–71. [Google Scholar]
- Christian, A.E.; Haynes, M.P.; Phillips, M.C.; Rothblat, G.H. Use of cyclodextrins for manipulating cellular cholesterol content. J. Lipid Res. 1997, 38, 2264–2272. [Google Scholar] [CrossRef]
- Koizume, S.; Ito, S.; Yoshioka, Y.; Kanayama, T.; Nakamura, Y.; Yoshihara, M.; Yamada, R.; Ochiya, T.; Ruf, W.; Miyagi, E.; et al. High-level secretion of tissue factor-rich extracellular vesicles from ovarian cancer cells mediated by filamin-A and protease-activated receptors. Thromb. Haemost. 2016, 115, 299–310. [Google Scholar]
- Ni, K.; Wang, C.; Carnino, J.M.; Jin, Y. The Evolving Role of Caveolin-1: A Critical Regulator of Extracellular Vesicles. Med. Sci. 2020, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.D.; Chen, J.; Castellon, M.; Mao, M.; Raj, J.U.; Comhair, S.; Erzurum, S.; Silva, C.L.; Machado, R.F.; Bonini, M.G.; et al. Injury-Induced Shedding of Extracellular Vesicles Depletes Endothelial Cells of Cav-1 (Caveolin-1) and Enables TGF-β (Transforming Growth Factor-β)-Dependent Pulmonary Arterial Hypertension. Arter. Thromb. Vasc. Biol. 2019, 39, 1191–1202. [Google Scholar] [CrossRef]
- Lee, H.; Li, C.; Zhang, Y.; Zhang, D.; Otterbein, L.E.; Jin, Y. Caveolin-1 selectively regulates microRNA sorting into microvesicles after noxious stimuli. J. Exp. Med. 2019, 216, 2202–2220. [Google Scholar] [CrossRef]
- Banfi, C.; Brioschi, M.; Barcella, S.; Pignieri, A.; Parolari, A.; Biglioli, P.; Tremoli, E.; Mussoni, L. Tissue factor induction by protease-activated receptor 1 requires intact caveolin-enriched membrane microdomains in human endothelial cells. J. Thromb. Haemost. 2007, 5, 2437–2444. [Google Scholar] [CrossRef]
- Felicetti, F.; Parolini, I.; Bottero, L.; Fecchi, K.; Errico, M.C.; Raggi, C.; Biffoni, M.; Spadaro, F.; Lisanti, M.P.; Sargiacomo, M.; et al. Caveolin-1 tumor-promoting role in human melanoma. Int. J. Cancer 2009, 125, 1514–1522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, F.; Ma, C.; Zhou, T.; Yandong, Z.; Luo, Q.; Geng, L.; Ding, L.; Zhang, Y.; Zhang, L.; Li, N.; et al. Exosomes derived from gemcitabine-resistant cells transfer malignant phenotypic traits via delivery of miRNA-222-3p. Mol. Cancer 2017, 16, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R.F. Routes and mechanisms of extracellular vesicle uptake. J. Extracell. Vesicles 2014, 3, 24641. [Google Scholar] [CrossRef] [Green Version]
- Yue, K.-Y.; Zhang, P.-R.; Zheng, M.-H.; Cao, X.-L.; Cao, Y.; Zhang, Y.-Z.; Zhang, Y.-F.; Wu, H.-N.; Lu, Z.-H.; Liang, L.; et al. Neurons can upregulate Cav-1 to increase intake of endothelial cells-derived extracellular vesicles that attenuate apoptosis via miR-1290. Cell Death Dis. 2019, 10, 869. [Google Scholar] [CrossRef]
- Verdera, H.C.; Gitz-Francois, J.J.; Schiffelers, R.M.; Vader, P. Cellular uptake of extracellular vesicles is mediated by clathrin-independent endocytosis and macropinocytosis. J. Control. Release 2017, 266, 100–108. [Google Scholar] [CrossRef]
- Delenclos, M.; Trendafilova, T.; Mahesh, D.; Baine, A.M.; Moussaud, S.; Yan, I.K.; Patel, T.; McLean, P.J. Investigation of Endocytic Pathways for the Internalization of Exosome-Associated Oligomeric Alpha-Synuclein. Front. Neurosci. 2017, 11, 172. [Google Scholar] [CrossRef] [Green Version]
- Andrews, A.M.; Rizzo, V. Microparticle-Induced Activation of the Vascular Endothelium Requires Caveolin-1/Caveolae. PLoS ONE 2016, 11, e0149272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svensson, K.J.; Christianson, H.C.; Wittrup, A.; Bourseau-Guilmain, E.; Lindqvist, E.; Svensson, L.M.; Mörgelin, M.; Belting, M. Exosome Uptake Depends on ERK1/2-Heat Shock Protein 27 Signaling and Lipid Raft-mediated Endocytosis Negatively Regulated by Caveolin-1. J. Biol. Chem. 2013, 288, 17713–17724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.L.; Cai, W.S.; Shen, F.; Feng, Z.; Zhu, G.H.; Cao, J.; Xu, B. Protease-activated receptor-2 modulates hepatic stellate cell collagen release and apoptotic status. Arch. Biochem. Biophys. 2014, 545, 162–166. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madkhali, Y.; Rondon, A.M.R.; Featherby, S.; Maraveyas, A.; Greenman, J.; Ettelaie, C. Factor VIIa Regulates the Level of Cell-Surface Tissue Factor through Separate but Cooperative Mechanisms. Cancers 2021, 13, 3718. https://doi.org/10.3390/cancers13153718
Madkhali Y, Rondon AMR, Featherby S, Maraveyas A, Greenman J, Ettelaie C. Factor VIIa Regulates the Level of Cell-Surface Tissue Factor through Separate but Cooperative Mechanisms. Cancers. 2021; 13(15):3718. https://doi.org/10.3390/cancers13153718
Chicago/Turabian StyleMadkhali, Yahya, Araci M. R. Rondon, Sophie Featherby, Anthony Maraveyas, John Greenman, and Camille Ettelaie. 2021. "Factor VIIa Regulates the Level of Cell-Surface Tissue Factor through Separate but Cooperative Mechanisms" Cancers 13, no. 15: 3718. https://doi.org/10.3390/cancers13153718