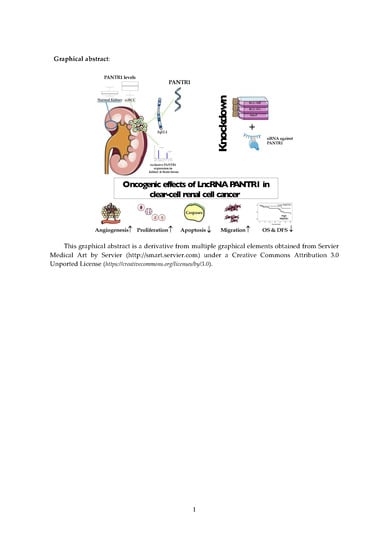
Long Non-Coding RNA PANTR1 is Associated with Poor Prognosis and Influences Angiogenesis and Apoptosis in Clear-Cell Renal Cell Cancer
Abstract
:1. Introduction
2. Results
2.1. PANTR1 is Up-Regulated in RCC Cancer Cells and Tissue and is Associated with Poor Survival of RCC Patients
2.2. Reduced Levels of PANTR1 Decrease Cellular Growth
2.3. PANTR1 Silencing Induces Apoptosis
2.4. PANTR1 Knock-Down Decreases Key Parameters of Angiogenesis and Endothelial Cell Migration
3. Discussion
4. Materials and Methods
4.1. Analysis of Gene Expression and Survival Data
4.2. In Situ Hybridization
4.3. Cell Culture
4.4. Transient Transfection with siRNAs
4.5. RNA Isolation and qRT-PCR
4.6. Caspase 3/7 Apoptosis Assay
4.7. Western Blot
4.8. WST-1 Proliferation Assay
4.9. Tube Formation Assay
4.10. Endothelial Migration Assay
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ljungberg, B.; Albiges, L.; Abu-ghanem, Y.; Bensalah, K.; Giles, R.H.; Hofmann, F.; Dabestani, S.; Ferna, S.; Hora, M.; Kuczyk, M.A.; et al. European Association of Urology Guidelines on Renal Cell Carcinoma: The 2019 Update. Eur. Urol. 2019, 5, 799–810. [Google Scholar] [CrossRef] [PubMed]
- Kroeger, N.; Xie, W.; Lee, J.L.; Bjarnason, G.A.; Knox, J.J.; MacKenzie, M.J.; Wood, L.; Srinivas, S.; Vaishamayan, U.N.; Rha, S.Y.; et al. Metastatic non-clear cell renal cell carcinoma treated with targeted therapy agents: Characterization of survival outcome and application of the International mRCC Database Consortium criteria. Cancer 2013, 119, 2999–3006. [Google Scholar] [CrossRef] [PubMed]
- Courtney, K.D.; Ma, Y.; de Leon, A.D.; Christie, A.; Xie, Z.; Woolford, L.; Singla, N.; Joyce, A.; Hill, H.; Madhuranthakam, A.J.; et al. HIF-2 complex dissociation, target inhibition, and acquired resistance with PT2385, a first-in-class HIF-2 inhibitor, in patients with clear cell renal cell carcinoma. Clin. Cancer Res. 2020, 26, 793–803. [Google Scholar] [CrossRef] [PubMed]
- Pichler, M.; Hutterer, G.C.; Chromecki, T.F.; Jesche, J.; Kampel-Kettner, K.; Groselj-Strele, A.; Hoefler, G.; Pummer, K.; Zigeuner, R. Comparison of the 2002 and 2010 TNM classification systems regarding outcome prediction in clear cell and papillary renal cell carcinoma. Histopathology 2013, 62, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Pichler, M.; Hutterer, G.C.; Chromecki, T.F.; Jesche, J.; Kampel-Kettner, K.; Eberhard, K.; Hoefler, G.; Pummer, K.; Zigeuner, R. Trends of stage, grade, histology and tumour necrosis in renal cell carcinoma in a European centre surgical series from 1984 to 2010. J. Clin. Pathol. 2012, 65, 721–724. [Google Scholar] [CrossRef]
- Pichler, M.; Hutterer, G.C.; Stoeckigt, C.; Chromecki, T.F.; Stojakovic, T.; Golbeck, S.; Eberhard, K.; Gerger, A.; Mannweiler, S.; Pummer, K.; et al. Validation of the pre-treatment neutrophil-lymphocyte ratio as a prognostic factor in a large European cohort of renal cell carcinoma patients. Br. J. Cancer 2013, 108, 901–907. [Google Scholar] [CrossRef] [Green Version]
- Pichler, M.; Hutterer, G.C.; Chromecki, T.F.; Jesche, J.; Groselj-Strele, A.; Kampel-Kettner, K.; Pummer, K.; Zigeuner, R. Prognostic value of the leibovich prognosis score supplemented by vascular invasion for clear cell renal cell carcinoma. J. Urol. 2012, 187, 834–839. [Google Scholar] [CrossRef]
- Pichler, M.; Hutterer, G.; Chromecki, T.; Jesche, J.; Kampel-Kettner, K.; Pummer, K.; Zigeuner, R. Renal cell carcinoma stage migration in a single European centre over 25 years: Effects on 5- and 10-year metastasis-free survival. Int. Urol. Nephrol. 2012, 44, 997–1004. [Google Scholar] [CrossRef]
- Pichler, M.; Hutterer, G.C.; Stojakovic, T.; Mannweiler, S.; Pummer, K.; Zigeuner, R. High plasma fibrinogen level represents an independent negative prognostic factor regarding cancer-specific, metastasis-free, as well as overall survival in a European cohort of non-metastatic renal cell carcinoma patients. Br. J. Cancer 2013, 109, 1123–1129. [Google Scholar] [CrossRef] [Green Version]
- Pichler, M.; Hutterer, G.C.; Chromecki, T.F.; Pummer, K.; Mannweiler, S.; Zigeuner, R. Presence and extent of histological tumour necrosis is an adverse prognostic factor in papillary type 1 but not in papillary type 2 renal cell carcinoma. Histopathology 2013, 62, 219–228. [Google Scholar] [CrossRef]
- Seles, M.; Posch, F.; Pichler, G.P.; Gary, T.; Pummer, K.; Zigeuner, R.; Hutterer, G.C.; Pichler, M. Blood Platelet Volume Represents a Novel Prognostic Factor in Patients with Nonmetastatic Renal Cell Carcinoma and Improves the Predictive Ability of Established Prognostic Scores. J. Urol. 2017, 198, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Klatte, T.; Rossi, S.H.; Stewart, G.D. Prognostic factors and prognostic models for renal cell carcinoma: A literature review. World J. Urol. 2018, 36, 1943–1952. [Google Scholar] [CrossRef] [PubMed]
- Ling, H.; Vincent, K.; Pichler, M.; Fodde, R.; Berindan-Neagoe, I.; Slack, F.J.; Calin, G. a Junk DNA and the long non-coding RNA twist in cancer genetics. Oncogene 2015, 34, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, Y.; Fullwood, M.J. Roles, Functions, and Mechanisms of Long Non-coding RNAs in Cancer. Genomics Proteomics Bioinformatics 2016, 14, 42–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Wu, Z.; Fu, X.; Han, W. Long Noncoding RNAs: Insights from Biological Features and Functions to Diseases. Med. Res. Rev. 2013, 33, 517–553. [Google Scholar] [CrossRef] [PubMed]
- Seles, M.; Hutterer, G.; Kiesslich, T.; Pummer, K.; Berindan-Neagoe, I.; Perakis, S.; Schwarzenbacher, D.; Stotz, M.; Gerger, A.; Pichler, M. Current Insights into Long Non-Coding RNAs in Renal Cell Carcinoma. Int. J. Mol. Sci. 2016, 17, 573. [Google Scholar] [CrossRef] [Green Version]
- Martens-Uzunova, E.S.; Böttcher, R.; Croce, C.M.; Jenster, G.; Visakorpi, T.; Calin, G.A. Long noncoding RNA in prostate, bladder, and kidney cancer. Eur. Urol. 2014, 65, 1140–1151. [Google Scholar] [CrossRef]
- Kumar, S.; Rathkolb, B.; Kemter, E.; Sabrautzki, S.; Michel, D.; Adler, T.; Becker, L.; Beckers, J.; Busch, D.H.; Garrett, L.; et al. Generation and Standardized, Systemic Phenotypic Analysis of Pou3f3 L423P Mutant Mice. PLoS ONE 2016, 11, e0150472. [Google Scholar]
- Li, W.; Wu, X.; She, W. LncRNA POU3F3 promotes cancer cell migration and invasion in nasopharyngeal carcinoma by up-regulating TGF-β1. Biosci. Rep. 2019, 39, BSR20181632. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Li, Y.; Wang, D.; Meng, Q. Linc-POU3F3 is overexpressed in hepatocellular carcinoma and regulates cell proliferation, migration and invasion. Biomed. Pharmacother. 2018, 105, 683–689. [Google Scholar] [CrossRef]
- Shan, T.-D.; Xu, J.-H.; Yu, T.; Li, J.-Y.; Zhao, L.-N.; Ouyang, H.; Luo, S.; Lu, X.-J.; Huang, C.-Z.; Lan, Q.-S.; et al. Knockdown of linc-POU3F3 suppresses the proliferation, apoptosis, and migration resistance of colorectal cancer. Oncotarget 2016, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, Y.S.; Wang, X.W.; Zhou, X.L.; Liu, Z.H.; Yang, T.X.; Shi, W.H.; Xie, H.W.; Lv, J.; Wu, Q.Q.; Cao, X.F. Identification of the long non-coding RNA POU3F3 in plasma as a novel biomarker for diagnosis of esophageal squamous cell carcinoma. Mol. Cancer 2015, 14, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, H.-L.; Hu, G.-W.; Chen, Y.; Liu, Y.; Tu, W.; Lu, Y.-M.; Wu, L.; Xu, G.-H. Glioma cells promote angiogenesis through the release of exosomes containing long non-coding RNA POU3F3. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 959–972. [Google Scholar] [PubMed]
- Wan, X.; Xiang, J.; Zhang, Q.; Bian, C. Long noncoding RNA POU3F3 promotes cancer cell proliferation in prostate carcinoma by upregulating rho-associated protein kinase 1. J. Cell. Biochem. 2019, 120, 8195–8200. [Google Scholar] [CrossRef]
- Guo, H.; Wu, L.; Yang, Q.; Ye, M.; Zhu, X. Functional linc-POU3F3 is overexpressed and contributes to tumorigenesis in glioma. Gene 2015, 554, 114–119. [Google Scholar] [CrossRef]
- Kaufmann, S.H.; Desnoyers, S.; Ottaviano, Y.; Davidson, N.E.; Poirier, G.G. Specific Proteolytic Cleavage of Poly(ADP-ribose) Polymerase: An Early Marker of Chemotherapy-induced Apoptosis. Cancer Res. 1993, 53, 3976–3985. [Google Scholar]
- Lamalice, L.; Le Boeuf, F.; Huot, J. Endothelial cell migration during angiogenesis. Circ. Res. 2007, 100, 782–794. [Google Scholar] [CrossRef]
- Claesson-Welsh, L.; Welsh, M. VEGFA and tumour angiogenesis. J. Intern. Med. 2013, 273, 114–127. [Google Scholar] [CrossRef]
- Garg, M.; Kanojia, D.; Okamoto, R.; Jain, S.; Jain, V.; Chien, W.; Sampath, A.; Ding, L.W.; Xuan, M.; Said, J.W.; et al. Laminin-5γ-2 (LAMC2) is highly expressed in anaplastic thyroid carcinoma and is associated with tumor progression, migration, and invasion by modulating signaling of EGFR. J. Clin. Endocrinol. Metab. 2014, 99, 62–72. [Google Scholar] [CrossRef] [Green Version]
- Sauvageau, M.; Goff, L.A.; Lodato, S.; Bonev, B.; Groff, A.F.; Gerhardinger, C.; Sanchez-Gomez, D.B.; Hacisuleyman, E.; Li, E.; Spence, M.; et al. Multiple knockout mouse models reveal lincRNAs are required for life and brain development. Elife 2013, 2013, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Nakai, S.; Sugitani, Y.; Sato, H.; Ito, S.; Miura, Y.; Ogawa, M.; Nishi, M.; Jishage, K.I.; Minowa, O.; Noda, T. Crucial roles fo Brn1 in distal tubule formation and function in mouse kidney. Development 2003, 130, 4751–4759. [Google Scholar] [CrossRef] [Green Version]
- Dheedene, A.; Maes, M.; Vergult, S.; Menten, B. A de novo POU3F3 Deletion in a Boy with Intellectual Disability and Dysmorphic. Mol. Syndromol. 2014, 5, 32–35. [Google Scholar] [PubMed] [Green Version]
- Blok, L.S.; Kleefstra, T.; Venselaar, H.; Maas, S.; Kroes, H.Y.; Lachmeijer, A.M.A.; Van Gassen, K.L.I.; Firth, H.V.; Tomkins, S.; Bodek, S.; et al. De Novo Variants Disturbing the Transactivation Capacity of POU3F3 Cause a Characteristic Neurodevelopmental Disorder. Am. J. Hum. Genet. 2019, 1–10. [Google Scholar]
- Ljungberg, B.; Albiges, L.; Bensalah, K.; Vice-chair, A.B.; Advocate, R.H.G.P.; Hora, M.; Kuczyk, M.A.; Lam, T.; Marconi, L.; Merseburger, A.S.; et al. EAU Guidelines on Renal Cell Carcinoma. Eur. Urol. 2019, 23, 799–810. [Google Scholar]
- Schanza, L.M.; Seles, M.; Stotz, M.; Fosselteder, J.; Hutterer, G.C.; Pichler, M.; Stiegelbauer, V. MicroRNAs associated with Von Hippel-Lindau pathway in renal cell carcinoma: A comprehensive review. Int. J. Mol. Sci. 2017, 18, 2495. [Google Scholar] [CrossRef] [Green Version]
- Shen, C.; Kaelin, W.G., Jr. The VHL/HIF axis in clear cell renal carcinoma. Semin. Cancer Biol. 2013, 23, 18–25. [Google Scholar] [CrossRef] [Green Version]
- Moch, H.; Cubilla, A.L.; Humphrey, P.A.; Reuter, V.E.; Ulbright, T.M. The 2016 WHO Classification of Tumours of the Urinary System and Male Genital Organs—Part A: Renal, Penile, and Testicular Tumours. Eur. Urol. 2016, 70, 93–105. [Google Scholar] [CrossRef]
- Fernández-Pello, S.; Hofmann, F.; Tahbaz, R.; Marconi, L.; Lam, T.B.; Albiges, L.; Bensalah, K.; Canfield, S.E.; Dabestani, S.; Giles, R.H.; et al. A Systematic Review and Meta-analysis Comparing the Effectiveness and Adverse Effects of Different Systemic Treatments for Non-clear Cell Renal Cell Carcinoma. Eur. Urol. 2017, 71, 426–436. [Google Scholar] [CrossRef]
- Li, W.; Zheng, J.; Deng, J.; You, Y.; Wu, H.; Li, N.; Lu, J.; Zhou, Y. Increased levels of the long intergenic non-protein coding RNA POU3F3 promote DNA methylation in esophageal squamous cell carcinoma cells. Gastroenterology 2014, 146, 1714–1726. [Google Scholar] [CrossRef]
- Chang, S.; Sun, L.; Feng, G. SP1-mediated long noncoding RNA POU3F3 accelerates the cervical cancer through miR-127-5p/FOXD1. Biomed. Pharmacother. 2019, 117. [Google Scholar] [CrossRef]
- Xiong, G.; Yang, L.; Chen, Y.; Fan, Z. Linc-POU3F3 promotes cell proliferation in gastric cancer via increasing T-reg distribution. Am. J. Transl. Res. 2015, 7, 2262–2269. [Google Scholar]
- Yang, J.; Meng, X.; Yu, Y.; Pan, L.; Zheng, Q.; Lin, W. LncRNA POU3F3 promotes proliferation and inhibits apoptosis of cancer cells in triple-negative breast cancer by inactivating caspase 9. Biosci. Biotechnol. Biochem. 2019, 83, 1117–1123. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Review Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerlinger, M.; Rowan, A.J.; Horswell, S.; Larkin, J.; Endesfelder, D.; Gronroos, E.; Martinez, P.; Matthews, N.; Stewart, A.; Tarpey, P.; et al. Intratumor Heterogeneity and Branched Evolution Revealed by Multiregion Sequencing. N. Engl. J. Med. 2012, 366, 883–892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takyar, S.; Diaz, J.; Sehgal, M.; Sapunar, F.; Pandha, H. First-line therapy for treatment-naive patients with advanced/metastatic renal cell carcinoma: A systematic review of published randomized controlled trials. Anticancer Drugs 2016, 27, 383–397. [Google Scholar] [CrossRef]
- Lai, Y.; Zeng, T.; Liang, X.; Wu, W.; Zhong, F.; Wu, W. Cell death-related molecules and biomarkers for renal cell carcinoma targeted therapy. Cancer Cell Int. 2019, 19, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liou, J.S.; Wu, Y.C.; Yen, W.Y.; Tang, Y.S.; Kakadiya, R.B.; Su, T.L.; Yih, L.H. Inhibition of autophagy enhances DNA damage-induced apoptosis by disrupting CHK1-dependent S phase arrest. Toxicol. Appl. Pharmacol. 2014, 278, 249–258. [Google Scholar] [CrossRef]
- Masoud, G.N.; Li, W. HIF-1α pathway: Role, regulation and intervention for cancer therapy. Acta Pharm. Sin. B 2015, 5, 378–389. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.; Du, C.; Ji, D.; Xi, J.; Gu, J. Overexpression of LAMC2 predicts poor prognosis in colorectal cancer patients and promotes cancer cell proliferation, migration, and invasion. Tumor Biol. 2017, 39. [Google Scholar] [CrossRef] [Green Version]
- Pei, Y.F.; Liu, J.; Cheng, J.; Wu, W.D.; Liu, X.Q. Silencing of LAMC2 Reverses Epithelial-Mesenchymal Transition and Inhibits Angiogenesis in Cholangiocarcinoma via Inactivation of the Epidermal Growth Factor Receptor Signaling Pathway. Am. J. Pathol. 2019, 189, 1637–1653. [Google Scholar] [CrossRef]
- Zhang, D.; Guo, H.; Feng, W.; Qiu, H. LAMC2 regulated by microRNA-125a-5p accelerates the progression of ovarian cancer via activating p38 MAPK signalling. Life Sci. 2019, 232, 116648. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, H.; Itoh, F.; Iku, S.; Hosokawa, M.; Imai, K. Expression of the γ2 chain of laminin-5 at the invasive front is associated with recurrence and poor prognosis in human esophageal squamous cell carcinoma. Clin. Cancer Res. 2001, 7, 896–900. [Google Scholar] [PubMed]
- Morlando, M.; Fatica, A. Alteration of epigenetic regulation by long noncoding RNAs in cancer. Int. J. Mol. Sci. 2018, 19, 570. [Google Scholar] [CrossRef] [Green Version]
- Hanly, D.; Esteller, M.; Berdasco, M. Interplay between long non-coding RNAs and epigenetic machinery: Emerging targets in cancer? Phil. Trans. R. Soc. B 2018, 373, 20170074. [Google Scholar] [CrossRef] [PubMed]
- Kotake, Y.; Nakagawa, T.; Kitagawa, K.; Suzuki, S.; Liu, N.; Kitagawa, M.; Xiong, Y. Long non-coding RNA ANRIL is required for the PRC2 recruitment to and silencing of p15 INK4B tumor suppressor gene. Oncogene 2011, 30, 1956–1962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fazi, B.; Garbo, S.; Toschi, N.; Mangiola, A.; Lombari, M.; Sicari, D.; Battistelli, C.; Galardi, S.; Michienzi, A.; Trevisi, G.; et al. The lncRNA H19 positively affects the tumorigenic properties of glioblastoma cells and contributes to NKD1 repression through the recruitment of EZH2 on its promoter. Oncotarget 2018, 9, 15512–15525. [Google Scholar] [CrossRef] [PubMed]
- Portoso, M.; Ragazzini, R.; Brenčič, Ž.; Moiani, A.; Michaud, A.; Vassilev, I.; Wassef, M.; Servant, N.; Sargueil, B.; Margueron, R. PRC 2 is dispensable for HOTAIR -mediated transcriptional repression. EMBO J. 2017, 36, 981–994. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, F.; Ni, T.; Lu, W.; Lin, N.; Zhang, C.; Sun, Z. Linc-POU3F3 is overexpressed in in-stent restenosis patients and induces VSMC phenotypic transformation via POU3F3/miR-449a/KLF4 signaling pathway. Am. J. Transl. Res. 2019, 11, 4481–4490. [Google Scholar]
- Barth, D.A.; Slaby, O.; Klec, C.; Juracek, J.; Drula, R.; Calin, G.A.; Pichler, M. Current concepts of non-coding RNAs in the pathogenesis of non-clear cell renal cell carcinoma. Cancers 2019, 11, 1580. [Google Scholar] [CrossRef] [Green Version]
- HPA RNA-seq Normal Tissues Project ID: PRJEB4337. Available online: https://www.ncbi.nlm.nih.gov/gene/100506421 (accessed on 13 March 2020).
- Fagerberg, L.; Hallstrom, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the human tissue-specific expression by genome-wide integration of transcriptomics and antibody-based proteomics. Mol. Cell. Proteom. 2014, 13, 397–406. [Google Scholar] [CrossRef] [Green Version]
- van Roemeling, C.; Radisky, D.; Marlow, L.; Cooper, S.J.; Grebe, S.; Anastasiadis, P.; Tun, H.; Copland, J. Neuronal pentraxin 2 supports clear cell renal cell carcinoma by activating the AMPA-selective glutamate receptor-4 Christina. Cancer Res. 2015, 74, 4796–4810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gene Array Analysis of Clear Cell Renal Cell Carcinoma Tissue Versus Matched Normal Kidney Tissue in Humans. Available online: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE53757 (accessed on 20 March 2020).
- TCGA PanCancer Atlas. Available online: https://www.cbioportal.org/orgepia.cancer-pku.cn/ (accessed on 15 March 2020).
- TCGA Pan-Cancer Data Set derived by Kaplan Meier plotter. Available online: http://kmplot.com/analysis/index.php?p=service&cancer=pancancer_rnaseq (accessed on 10 March 2020).




| Parameter | Number of Patients | % |
|---|---|---|
| Total | 175 | 100 |
| Gender | ||
| Male | 108 | 61.7 |
| Female | 67 | 38.3 |
| Grading | ||
| G1 | 37 | 21.1 |
| G2 | 93 | 53.1 |
| G3 | 37 | 21.1 |
| G4 | 7 | 4 |
| GX | 1 | 0.6 |
| UICC TNM Stage | ||
| I | 124 | 70.9 |
| II | 22 | 12.6 |
| III | 27 | 15.4 |
| IV | 2 | 1.1 |
| Pathologic T stage | ||
| pT1 | 121 | 69.1 |
| pT2 | 23 | 13.1 |
| pT3 | 28 | 16.0 |
| pT4 | 2 | 1.1 |
| N/A | 1 | 0.6 |
| Lymph node metastasis | ||
| Yes | 2 | 1.1 |
| No | 173 | 98.9 |
| Disease Recurrence | ||
| Yes | 17 | 9.7 |
| No | 158 | 90.3 |
| Multivariate Analysis | ||
|---|---|---|
| Parameter | HR (95% CI) | p-Value |
| Age (continuous) | 1.02 (0.96–1.08) | 0.341 |
| Gender (female versus male) | 0.66 (0.23–1.85) | 0.428 |
| Tumor Grade (G1+2 versus G3+4) | 1.68 (0.94–2.99) | 0.079 |
| UICC TNM Stage (I/II versus III/IV | 2.38 (1.35–4.19) | 0.003 * |
| PANTR1 expression (low versus high group) | 4.30 (1.45–12.76) | 0.008 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seles, M.; Hutterer, G.C.; Foßelteder, J.; Svoboda, M.; Resel, M.; Barth, D.A.; Pichler, R.; Bauernhofer, T.; Zigeuner, R.E.; Pummer, K.; et al. Long Non-Coding RNA PANTR1 is Associated with Poor Prognosis and Influences Angiogenesis and Apoptosis in Clear-Cell Renal Cell Cancer. Cancers 2020, 12, 1200. https://doi.org/10.3390/cancers12051200
Seles M, Hutterer GC, Foßelteder J, Svoboda M, Resel M, Barth DA, Pichler R, Bauernhofer T, Zigeuner RE, Pummer K, et al. Long Non-Coding RNA PANTR1 is Associated with Poor Prognosis and Influences Angiogenesis and Apoptosis in Clear-Cell Renal Cell Cancer. Cancers. 2020; 12(5):1200. https://doi.org/10.3390/cancers12051200
Chicago/Turabian StyleSeles, Maximilian, Georg C. Hutterer, Johannes Foßelteder, Marek Svoboda, Margit Resel, Dominik A. Barth, Renate Pichler, Thomas Bauernhofer, Richard E. Zigeuner, Karl Pummer, and et al. 2020. "Long Non-Coding RNA PANTR1 is Associated with Poor Prognosis and Influences Angiogenesis and Apoptosis in Clear-Cell Renal Cell Cancer" Cancers 12, no. 5: 1200. https://doi.org/10.3390/cancers12051200