Trends in Treatment and Survival of Gallbladder Cancer in the Netherlands; Identifying Gaps and Opportunities from a Nation-Wide Cohort
Abstract
:1. Background
2. Methods
2.1. Patient Selection and Variable Definitions
2.2. Quality Control and Completeness of Data Assessment
2.3. Statistical Analysis
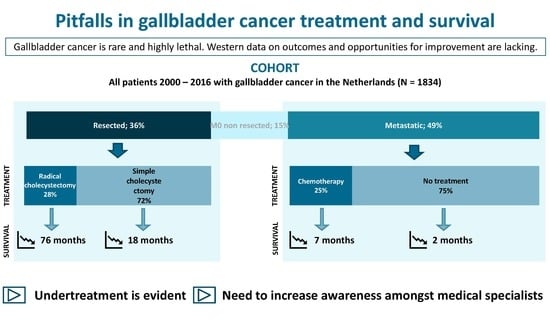
3. Results
3.1. Incidence and Patient and Tumor Characteristics
3.2. Treatment
3.3. Survival
3.4. Therapy and Survival
3.5. Prognostic Factors for Survival
3.6. Quality Control
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| ICD-03 code | Frequency (%) | |
| 8000 | Neoplasma | 310 (16.6) |
| 8001 | Tumor cells | 2 (0.1) |
| 8010 | Carcinoma, NOS | 50 (2.7) |
| 8012 | Large cell carcinoma NOS | 35 (1.9) |
| 8013 | Large cell neuroendocrine carcinoma | 6 (0.3) |
| 8020 | Carcinoma, undifferentiated, NOS | 5 (0.3) |
| 8030 | Giant cell and spindle cell carcinoma | 2 (0.1) |
| 8032 | Spindle cell carcinoma, NOS | 2 (0.1) |
| 8033 | Pseudosarcomatous carcinoma | 3 (0.2) |
| 8041 | Small cell carcinoma, NOS | 10 (0.5) |
| 8046 | Non-small cell carcinoma | 4 (0.2) |
| 8070 | Squamous cell carcinoma, NOS | 19 (1.0) |
| 8071 | Squamous cell carcinoma, keratinizing, NOS | 2 (0.1) |
| 8074 | Squamous cell carcinoma, spindle cell | 1 (0.1) |
| 8140 | Adenocarcinoma, NOS | 1171 (62.6) |
| 8144 | Adenocarcinoma, intestinal type | 21 (1.1) |
| 8160 | Cholangiocarcinoma | 6 (0.3) |
| 8163 | Pancreatobiliary-type carcinoma | 5 (0.3) |
| 8210 | Adenocarcinoma in adenomatous polyp | 7 (0.4) |
| 8211 | Tubular adenocarcinoma | 2 (0.1) |
| 8240 | Carcinoid tumor, NOS | 13 (0.7) |
| 8244 | Mixed adenoneuroendocrine carcinoma | 2 (0.1) |
| 8246 | Neuroendocrine carcinoma, NOS | 6 (0.3) |
| 8249 | Atypical carcinoid tumor | 2 (0.1) |
| 8260 | Papillary adenocarcinoma, NOS | 36 (1.9) |
| 8263 | Adenocarcinoma in tubolovillous adenoma | 3 (0.2) |
| 8310 | Clear cell adenocarcinoma, NOS | 3 (0.2) |
| 8312 | Renal cell carcinoma, NOS | 1 (0.1) |
| 8350 | Nonencapsulated sclerosing carcinoma | 1 (0.1) |
| 8480 | Mucinous adenocarcinoma | 31 (1.7) |
| 8481 | Mucin-producing adenocarcinoma | 44 (2.4) |
| 8490 | Signet ring cell carcinoma | 19 (1.0) |
| 8500 | Infiltrating duct carcinoma, NOS | 2 (0.1) |
| 8503 | Intraductal papillary adenocarcinoma with invasion | 4 (0.2) |
| 8560 | Adenosquamous carcinoma | 26 (1.4) |
| 8570 | Adenocarcinoma with squamous metaplasia | 1 (0.1) |
| 8574 | Adenocarcinoma with neuroendocrine differentiation | 10 (0.5) |
| 8575 | Metaplastic carcinoma, NOS | 1 (0.1) |
| 8576 | Hepatoid adenocarcinoma | 1 (0.1) |
| 8980 | Carcinosarcoma, NOS | 3 (0.2) |
Appendix B. Incidence of Gallbladder Cancer Per 100.000 Inhabitants in the Netherlands, 2005–2016

Appendix C. Survival of Gallbladder Cancer Patients by Disease Stage and Period of Diagnosis

References
- Are, C.; Ahmad, H.; Ravipati, A.; Croo, D.; Clarey, D.; Smith, L.; Price, R.R.; Butte, J.M.; Gupta, S.; Chaturvedi, A.; et al. Global epidemiological trends and variations in the burden of gallbladder cancer. J. Surg. Oncol. 2017, 115, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Hundal, R.; Shaffer, E.A. Gallbladder cancer: Epidemiology and outcome. Clin. Epidemiol. 2014, 6, 99–109. [Google Scholar] [PubMed]
- Piehler, J.M.; Crichlow, R.W. Primary carcinoma of the gallbladder. Surg. Gynecol. Obstet. 1978, 147, 929–942. [Google Scholar] [CrossRef]
- Bartlett, D.L.; Fong, Y.; Fortner, J.G.; Brennan, M.F.; Blumgart, L.H. Long-term results after resection for gallbladder cancer. Implications for staging and management. Ann. Surg. 1996, 224, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Hueman, M.T.; Vollmer, C.M., Jr.; Pawlik, T.M. Evolving treatment strategies for gallbladder cancer. Ann. Surg. Oncol. 2009, 16, 2101–2115. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, J.S.; Khan, M.R.; Ghias, K. DNA methylation as an epigenetic regulator of gallbladder cancer: An overview. Int. J. Surg. (London, England) 2018, 53, 178–183. [Google Scholar] [CrossRef]
- Larsson, S.C.; Wolk, A. Obesity and the risk of gallbladder cancer: A meta-analysis. Br. J. Cancer 2007, 96, 1457–1461. [Google Scholar] [CrossRef]
- de Martel, C.; Plummer, M.; Parsonnet, J.; van Doorn, L.J.; Franceschi, S. Helicobacter species in cancers of the gallbladder and extrahepatic biliary tract. Br. J. Cancer 2009, 100, 194–199. [Google Scholar] [CrossRef]
- Duffy, A.; Capanu, M.; Abou-Alfa, G.K.; Huitzil, D.; Jarnagin, W.; Fong, Y.; D’Angelica, M.; Dematteo, R.P.; Blumgart, L.H.; O’Reilly, E.M. Gallbladder cancer (GBC): 10-year experience at Memorial Sloan-Kettering Cancer Centre (MSKCC). J. Surg. Oncol. 2008, 98, 485–489. [Google Scholar] [CrossRef]
- Fong, Y.; Jarnagin, W.; Blumgart, L.H. Gallbladder cancer: Comparison of patients presenting initially for definitive operation with those presenting after prior noncurative intervention. Ann. Surg. 2000, 232, 557–569. [Google Scholar] [CrossRef]
- Cubertafond, P.; Gainant, A.; Cucchiaro, G. Surgical treatment of 724 carcinomas of the gallbladder. Results of the French Surgical Association Survey. Ann. Surg. 1994, 219, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Buettner, S.; Margonis, G.A.; Kim, Y.; Gani, F.; Ethun, C.G.; Poultsides, G.A.; Tran, T.; Idrees, K.; Isom, C.A.; Fields, R.C.; et al. Changing Odds of Survival Over Time among Patients Undergoing Surgical Resection of Gallbladder Carcinoma. Ann. Surg. Oncol. 2016, 23, 4401–4409. [Google Scholar] [CrossRef]
- He, X.D.; Li, J.J.; Liu, W.; Qu, Q.; Hong, T.; Xu, X.Q.; Li, B.L.; Wang, Y.; Zhao, H.T. Surgical procedure determination based on tumor-node-metastasis staging of gallbladder cancer. World J. Gastroenterol. 2015, 21, 4620–4626. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.M.; Hoshi, H.; Gibbs, J.F.; Iyer, R.; Javle, M.; Chu, Q.; Kuvshinoff, B. Gallbladder cancer: Defining the indications for primary radical resection and radical re-resection. Ann. Surg. Oncol. 2007, 14, 833–840. [Google Scholar] [CrossRef]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef] [Green Version]
- Edeline, J.; Benabdelghani, M.; Bertaut, A.; Watelet, J.; Hammel, P.; Joly, J.P.; Boudjema, K.; Fartoux, L.; Bouhier-Leporrier, K.; Jouve, J.L.; et al. Gemcitabine and Oxaliplatin Chemotherapy or Surveillance in Resected Biliary Tract Cancer (PRODIGE 12-ACCORD 18-UNICANCER GI): A Randomized Phase III Study. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2019, 37, 658–667. [Google Scholar] [CrossRef]
- Primrose, J.N.; Fox, R.P.; Palmer, D.H.; Malik, H.Z.; Prasad, R.; Mirza, D.; Anthony, A.; Corrie, P.; Falk, S.; Finch-Jones, M.; et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): A randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019, 20, 663–673. [Google Scholar] [CrossRef] [Green Version]
- Ausania, F.; Tsirlis, T.; White, S.A.; French, J.J.; Jaques, B.C.; Charnley, R.M.; Manas, D.M. Incidental pT2-T3 gallbladder cancer after a cholecystectomy: Outcome of staging at 3 months prior to a radical resection. HPB (Oxford) 2013, 15, 633–637. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, S.; Tomokuni, A.; Gotoh, K.; Takahashi, H.; Akita, H.; Marubashi, S.; Yamada, T.; Teshima, T.; Fukui, K.; Fujiwara, Y.; et al. A retrospective analysis of the clinical effects of neoadjuvant combination therapy with full-dose gemcitabine and radiation therapy in patients with biliary tract cancer. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2017, 43, 763–771. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.W.; Lamarca, A.; Goyal, L.; Barriuso, J.; Zhu, A.X. New Horizons for Precision Medicine in Biliary Tract Cancers. Cancer Discov. 2017, 7, 943–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casparie, M.; Tiebosch, A.T.M.G.; Burger, G.; Blauwgeers, H.; Van de Pol, A.; van Krieken, J.H.J.M.; Meijer, G.A. Pathology databanking and biobanking in The Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Cell. Oncol. Off. J. Int. Soc. Cell. Oncol. 2007, 29, 19–24. [Google Scholar]
- Schouten, L.J.; Hoppener, P.; van den Brandt, P.A.; Knottnerus, J.A.; Jager, J.J. Completeness of cancer registration in Limburg, The Netherlands. Int. J. Epidemiol. 1993, 22, 369–376. [Google Scholar] [CrossRef] [PubMed]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gotzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet (London, England) 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Greene, F.L.; Page, D.L.; Fleming, I.D.; Fritz, A.G.; Balch, C.M.; Haller, D.G.; Morrow, M. AJCC Cancer Staging Manual, 6th ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Edge, S.; Byrd, D.R.; Compton, C.C.; Fritz, A.G.; Greene, F.; Trotti, A. ACJJ Cancer Staging Handbook, 7th ed.; Springer: New York, NY, USA, 2010. [Google Scholar]
- Witjes, C.D.; Van Den Akker, S.A.; Visser, O.; Karim-Kos, H.E.; De Vries, E.; IJzermans, J.N.; Robert, A.; Coebergh, J.W.W.; Verhoef, C. Gallbladder cancer in the Netherlands: Incidence, treatment and survival patterns since 1989. Dig. Surg. 2012, 29, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Hari, D.M.; Howard, J.H.; Leung, A.M.; Chui, C.G.; Sim, M.S.; Bilchik, A.J. A 21-year analysis of stage I gallbladder carcinoma: Is cholecystectomy alone adequate? HPB: Off. J. Int. Hepato Pancreato Biliary Assoc. 2013, 15, 40–48. [Google Scholar] [CrossRef] [Green Version]
- Oh, T.G.; Chung, M.J.; Bang, S.; Park, S.W.; Chung, J.B.; Song, S.Y.; Choi, G.H.; Kim, K.S.; Lee, W.J.; Park, J.Y. Comparison of the sixth and seventh editions of the AJCC TNM classification for gallbladder cancer. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2013, 17, 925–930. [Google Scholar] [CrossRef] [PubMed]
- IKNL. Galweg- en Galblaascarcinoom 2.0; IKNL: Utrecht, The Netherlands, 2010. [Google Scholar]
- Koerkamp, B.G.; Jarnagin, W.R. Surgical Oncology: A Practical and Comprehensive Approach; Springer: New York, NY, USA, 2015; pp. 235–255. [Google Scholar]
- Lee, S.E.; Jang, J.Y.; Lim, C.S.; Kang, M.J.; Kim, S.W. Systematic review on the surgical treatment for T1 gallbladder cancer. World J. Gastroenterol. 2011, 17, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Goetze, T.O.; Paolucci, V. Immediate re-resection of T1 incidental gallbladder carcinomas: A survival analysis of the German Registry. Surg. Endosc. 2008, 22, 2462–2465. [Google Scholar] [CrossRef]
- Coburn, N.G.; Cleary, S.P.; Tan, J.C.; Law, C.H. Surgery for gallbladder cancer: A population-based analysis. J. Am. Coll. Surg. 2008, 207, 371–382. [Google Scholar] [CrossRef]
- Skipworth, J.R.; Olde Damink, S.W.; Imber, C.; Bridgewater, J.; Pereira, S.P.; Malago, M. Review article: Surgical, neo-adjuvant and adjuvant management strategies in biliary tract cancer. Aliment. Pharmacol. Ther. 2011, 34, 1063–1078. [Google Scholar] [CrossRef] [Green Version]
- Hennedige, T.P.; Neo, W.T.; Venkatesh, S.K. Imaging of malignancies of the biliary tract- an update. Cancer Imaging 2014, 14, 14. [Google Scholar] [PubMed] [Green Version]
- Javle, M.; Bekaii-Saab, T.; Jain, A.; Wang, Y.; Kelley, R.K.; Wang, K.; Kang, H.C.; Catenacci, D.; Ali, S.; Krishnan, S.; et al. Biliary cancer: Utility of next-generation sequencing for clinical management. Cancer 2016, 122, 3838–3847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, D.H.; Bekaii-Saab, T. Biliary cancer: Intrahepatic cholangiocarcinoma vs. extrahepatic cholangiocarcinoma vs. gallbladder cancers: Classification and therapeutic implications. J. Gastrointest. Oncol. 2017, 8, 293–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Cohort | Total (n = 1834) | Resected (n = 661) | Non-resected Non-metastatic (n = 278) | Metastatic (n = 895) |
|---|---|---|---|---|
| Patient and tumor characteristics | ||||
| Age | 71.1 (22–97) | 69.2 (27–97) | 74.3 (32–95) | 71.2 (22–96) |
| Male sex | 545 (29.1%) | 206 (31.2%) | 82 (29.5%) | 250 (27.9%) |
| Socioeconomic Status | ||||
| High | 501 (26.8%) | 183 (27.7%) | 82 (29.5%) | 229 (33.4%) |
| Medium | 741 (39.6%) | 253 (38.3%) | 110 (39.6%) | 367 (41.0%) |
| Low | 630 (33.7%) | 225 (34.0%) | 86 (30.9%) | 299 (33.4%) |
| Clinicopathologic T stage a | ||||
| T1 | 526 (28.1%) | 147 (22.6%) | 1 (0.4%) | 54 (8.5%) |
| T2 | 303 (45.8%) | 0 (0.0%) | 22 (2.5%) | |
| T3/T4 | 643 (34.3%) | 172 (26.2%) | 169 (60.8%) | 427 (47.7%) |
| TX | 496 (26.5%) | 38 (5.8%) | 13 (4.7%) | 302 (33.7%) |
| Unknown/missing | 207 (11.1%) | - | 95 (34.2%) | 90 (10.1%) |
| Clinicopathologic N stage a | ||||
| N0 | 674 (36.0%) | 140 (21.2%) | 62 (22.3%) | 237 (26.5%) |
| N1 | 432 (23.1%) | 123 (18.6%) | 74 (26.6%) | 331 (37.0%) |
| NX | 559 (29.9%) | 387 (58.5%) | 47 (16.9%) | 237 (26.5%) |
| Unknown/missing | 207 (11.1%) | 11 (1.7%) | 95 (34.2%) | 90 (10.1%) |
| Location synchronous metastases | ||||
| Liver | N/A | N/A | N/A | 350 (39.1%) |
| Peritoneal | N/A | N/A | N/A | 119 (13.3%) |
| Lymph node | N/A | N/A | N/A | 46 (5.1%) |
| Lung | N/A | N/A | N/A | 11 (1.2%) |
| Liver + peritoneum | N/A | N/A | N/A | 92 (10.3%) |
| Other | N/A | N/A | N/A | 22 (2.5%) |
| Multiple, other | N/A | N/A | N/A | 175 (19.6%) |
| Unknown/missing | N/A | N/A | N/A | 80 (8.9%) |
| Pathology confirmation of primary tumor(yes) | 1566 (83.7%) | 661 (100%) | 156 (56.1%) | 732 (81.8%) |
| Differentiation grade | ||||
| Well | N/A | 102 (15.4%) | N/A | N/A |
| Moderate | N/A | 209 (31.6%) | N/A | N/A |
| Poor | N/A | 157 (23.7%) | N/A | N/A |
| Not determined | N/A | 193 (29.2%) | N/A | N/A |
| Radicality | ||||
| R0 | N/A | 417 (63.1%) | N/A | N/A |
| R1 | N/A | 130 (19.7%) | N/A | N/A |
| R2 | N/A | 24 (3.6%) | N/A | N/A |
| Unclear | N/A | 90 (13.6%) | N/A | N/A |
| Group | N | Five-year Survival | Median OS, Months (95% CI) | Log Rank Test p Value |
|---|---|---|---|---|
| Total | 1895 | 13.2% | 5.5 (5.0–6.0) | |
| Resected non-metastatic | 661 | 34.2% | 23.7 (19.6–27.8) | |
| Adjuvant chemotherapy | 12 | 37.5% | 29.4 (21.4–37.5) | 0.521 |
| No adjuvant chemotherapy | 649 | 34.1% | 23.7 (19.4–27.6) | |
| T1b/T2 tumor, no radical surgery | 106 | 30.6% | 18.3 (13.8–22.7) | <0.001 |
| T1b/T2 tumor, radical surgery | 274 | 52.7% | 76.7 (43.0–110.3) | |
| Non-resected non-metastatic | 278 | 2.9% | 3.6 (3.1–4.1) | |
| No palliative chemotherapy | 257 | 3.0% | 3.5 (2.9–4.0) | 0.011 |
| Palliative chemotherapy | 21 | - | 7.7 (4.5–10.8 | |
| Metastatic | 895 | 1.3% | 2.9 (2.6–3.2) | |
| No palliative chemotherapy | 690 | 0.6% | 2.1 (1.9–2.4) | <0.001 |
| Palliative chemotherapy | 205 | 3.7% | 7.3 (6.4–8.2) |
| A. Prognostic factors for patients with resected gallbladder cancer. N = 661. | ||||||
| Characteristic | Univariable Cox Regression | Multivariable Cox Regression | ||||
| HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Grade | ||||||
| Well | 1 | 1 | ||||
| Moderate | 1.41 | 1.02–1.95 | 0.036 | 1.17 | 0.84–1.61 | 0.354 |
| Poor | 2.67 | 1.93–3.70 | <0.001 | 2.07 | 1.49–2.86 | <0.001 |
| Unknown | 1.45 | 1.05–1.99 | 0.023 | 1.74 | 1.26–2.41 | 0.001 |
| Sex | ||||||
| Female | 1 | |||||
| Male | 0.88 | 0.71–1.08 | 0.214 | |||
| Pathological T stage | ||||||
| T1 | 1 | 1 | ||||
| T2 | 1.77 | 1.35–2.32 | <0.001 | 1.58 | 1.19–2.10 | 0.001 |
| T3/T4 | 3.59 | 2.69–4.78 | <0.001 | 2.61 | 1.89–3.61 | <0.001 |
| Tx | 3.23 | 2.01–5.18 | <0.001 | 2.16 | 1.34–3.50 | 0.002 |
| Pathological N stage | ||||||
| N0 | 1 | 1 | ||||
| N1 | 2.96 | 2.13–4.12 | <0.001 | 1.95 | 1.39–2.74 | <0.001 |
| Nx | 2.48 | 1.86–3.31 | <0.001 | 1.86 | 1.46–2.66 | <0.001 |
| Radicality | ||||||
| R0 | 1 | 1 | ||||
| R1/R2 | 3.78 | 3.03–4.71 | <0.001 | 2.69 | 2.11–3.43 | <0.001 |
| Unclear | 1.60 | 1.20–2.14 | 0.001 | 1.48 | 1.10–1.98 | 0.009 |
| Adjuvant chemotherapy (yes) | 0.67 | 0.33–1.36 | 0.268 | |||
| Prior malignancy (yes) | 1.22 | 0.93–1.61 | 0.150 | |||
| Increasing age (years) | 1.04 | 1.03–1.05 | <0.001 | 1.04 | 1.03–1.05 | <0.001 |
| B. Prognostic factors for patients with metastatic gallbladder cancer. N = 895. | ||||||
| Characteristic | Univariable Cox Regression | Multivariable Cox Regression | ||||
| HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Grade | ||||||
| Well | 1 | |||||
| Moderately | 1.02 | 0.61–1.71 | 0.931 | |||
| Poor | 1.45 | 0.89–2.36 | 0.136 | |||
| Unknown | 1.85 | 1.16–2.97 | 0.010 | |||
| Sex | ||||||
| Female | 1 | |||||
| Male | 0.88 | 0.71–1.08 | 0.214 | |||
| Clinical T stage | ||||||
| T1/T2 | 1 | 1 | ||||
| T3/T4 | 2.01 | 1.57–2.58 | <0.001 | 1.33 | 1.02–1.73 | 0.036 |
| Tx | 1.82 | 1.41–2.35 | <0.001 | 1.33 | 1.02–1.74 | 0.035 |
| Unknown | 3.94 | 2.88–5.39 | <0.001 | 2.22 | 1.57–3.15 | <0.001 |
| Clinical N stage | ||||||
| N0 | 1 | 1 | ||||
| N1 | 1.28 | 1.07–1.50 | 0.006 | 1.21 | 1.02–1.44 | 0.031 |
| Nx | 1.50 | 1.25–1.80 | <0.001 | 1.54 | 1.28–1.86 | <0.001 |
| Unknown | 2.70 | 2.11–3.47 | <0.001 | ** | ||
| Supportive therapy (yes) | 1.07 | 0.90–1.27 | 0.443 | |||
| Palliative chemotherapy (yes) | 0.46 | 0.39–0.54 | <0.001 | 0.47 | 0.39–0.55 | <0.001 |
| Prior malignancy (yes) | 0.93 | 0.80–1.08 | 0.358 | |||
| Increasing age (year) | 1.03 | 1.03–1.04 | <0.001 | 1.02 | 1.01–1.03 | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Savornin Lohman, E.; de Bitter, T.; Verhoeven, R.; van der Geest, L.; Hagendoorn, J.; Haj Mohammad, N.; Daams, F.; Klümpen, H.-J.; van Gulik, T.; Erdmann, J.; et al. Trends in Treatment and Survival of Gallbladder Cancer in the Netherlands; Identifying Gaps and Opportunities from a Nation-Wide Cohort. Cancers 2020, 12, 918. https://doi.org/10.3390/cancers12040918
de Savornin Lohman E, de Bitter T, Verhoeven R, van der Geest L, Hagendoorn J, Haj Mohammad N, Daams F, Klümpen H-J, van Gulik T, Erdmann J, et al. Trends in Treatment and Survival of Gallbladder Cancer in the Netherlands; Identifying Gaps and Opportunities from a Nation-Wide Cohort. Cancers. 2020; 12(4):918. https://doi.org/10.3390/cancers12040918
Chicago/Turabian Stylede Savornin Lohman, Elise, Tessa de Bitter, Rob Verhoeven, Lydia van der Geest, Jeroen Hagendoorn, Nadia Haj Mohammad, Freek Daams, Heinz-Josef Klümpen, Thomas van Gulik, Joris Erdmann, and et al. 2020. "Trends in Treatment and Survival of Gallbladder Cancer in the Netherlands; Identifying Gaps and Opportunities from a Nation-Wide Cohort" Cancers 12, no. 4: 918. https://doi.org/10.3390/cancers12040918