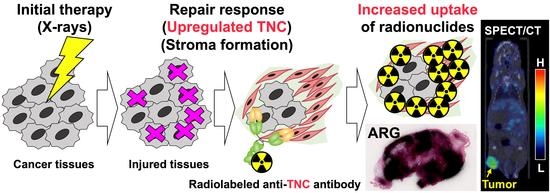
Proof of Concept Study for Increasing Tenascin-C-Targeted Drug Delivery to Tumors Previously Subjected to Therapy: X-Irradiation Increases Tumor Uptake
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Analysis of TNC Expression in Tumors Treated with X-Irradiation
2.2. Biodistribution of 111In-Labeled Antibodies
2.3. Single-Photon Emission Computed Tomography and Computed Tomography (SPECT/CT) with 111In-Labeled Antibodies
2.4. Autoradiography
2.5. Immunohistochemistry to Compare Antibodies 3–6 and 12–2–7
3. Discussion
4. Materials and Methods
4.1. Antibody
4.2. Cells
4.3. Cell Binding of 111In-Labeled Anti-TNC 3–6
4.4. Mouse Model of Subcutaneous Tumors
4.5. Immunohistochemistry with Anti-TNC Antibodies
4.6. Radiolabeling of Antibodies
4.7. Biodistribution of 111In-Labeled Antibodies
4.8. SPECT/CT with 111In-Labeled Antibodies
4.9. Autoradiography
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Tuli, R.; Shinde, A.; Hendifar, A.E. Meta-analyses of treatment standards for pancreatic cancer. Mol. Clin. Oncol. 2016, 4, 315–325. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Siegel, R.L.; Lin, C.C.; Mariotto, A.B.; Kramer, J.L.; Rowland, J.H.; Stein, K.D.; Alteri, R.; Jemal, A. Cancer treatment and survivorship statistics, 2016. CA Cancer J. Clin. 2016, 66, 271–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brellier, F.; Chiquet-Ehrismann, R. How do tenascins influence the birth and life of a malignant cell? J. Cell Mol. Med. 2012, 16, 32–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Midwood, K.S.; Chiquet, M.; Tucker, R.P.; Orend, G. Tenascin-C at a glance. J. Cell Sci. 2016, 129, 4321–4327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Midwood, K.S.; Orend, G. The role of tenascin-C in tissue injury and tumorigenesis. J. Cell Commun. Signal 2009, 3, 287–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koyama, Y.; Norose, K.; Kusubata, M.; Irie, S.; Kusakabe, M. Differential expression of tenascin in the skin during hapten-induced dermatitis. Histochem. Cell Biol. 1996, 106, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, P.; Kusakabe, M.; Mori, M. Tenascin in human neoplasia. Int. J. Oncol. 1996, 8, 741–755. [Google Scholar] [CrossRef]
- Tarin, D. The Cancer Stroma and Its Relevance to Tumor Survival and Treatment. In Cancer Drug Delivery Systems Based on the Tumor Microenvironment; Matsumura, Y., Tarin, D., Eds.; Springer: Tokyo, Japan, 2020; pp. 3–22. ISBN 9784431568780. [Google Scholar]
- Yoshida, T.; Akatsuka, T.; Imanaka-Yoshida, K. Tenascin-C and integrins in cancer. Cell Adhes. Migr. 2015, 9, 96–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reilly, R.M. The Radiochemistry of Monoclonal Antibodies and Peptides. In Monoclonal Antibody and Peptide-Targeted Radiotherapy of Cancer; Reilly, R.M., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 39–100. ISBN 9780470613214. [Google Scholar]
- Matsumoto, K.; Hiraiwa, N.; Yoshiki, A.; Ohnishi, M.; Kusakabe, M. Tenascin-C expression and splice variant in habu snake venom-induced glomerulonephritis. Exp. Mol. Pathol. 2002, 72, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Giblin, S.P.; Midwood, K.S. Tenascin-C: Form versus function. Cell Adhes. Migr. 2015, 9, 48–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foley, K.; Muth, S.; Jaffee, E.; Zheng, L. Hedgehog signaling stimulates Tenascin C to promote invasion of pancreatic ductal adenocarcinoma cells through Annexin A2. Cell Adhes. Migr. 2017, 11, 514–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sudo, H.; Tsuji, A.B.; Sugyo, A.; Kurosawa, G.; Kurosawa, Y.; Alexander, D.; Tsuda, H.; Saga, T.; Higashi, T. Radiolabeled Human Monoclonal Antibody 067-213 has the Potential for Noninvasive Quantification of CD73 Expression. Int. J. Mol. Sci. 2020, 21, 2304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugyo, A.; Tsuji, A.B.; Sudo, H.; Koizumi, M.; Ukai, Y.; Kurosawa, G.; Kurosawa, Y.; Saga, T.; Higashi, T. Efficacy Evaluation of Combination Treatment Using Gemcitabine and Radioimmunotherapy with 90Y-Labeled Fully Human Anti-CD147 Monoclonal Antibody059–053 in a BxPC-3 Xenograft Mouse Model of Refractory Pancreatic Cancer. Int. J. Mol. Sci. 2018, 19, 2979. [Google Scholar] [CrossRef] [PubMed] [Green Version]









| Tissue/Organ | 30 Min | Day 1 | Day 2 | Day 4 |
|---|---|---|---|---|
| 0 Gy | ||||
| Blood | 40.6 ± 3.1 | 17.8 ± 1.3 | 15.7 ± 1.9 | 13.3 ± 1.3 |
| Lung | 13.8 ± 2.8 | 7.8 ± 0.6 | 6.6 ± 1.3 | 5.9 ± 1.1 |
| Liver | 12.1 ± 0.9 | 9.7 ± 1.1 | 8.7 ± 0.8 | 8.1 ± 1.2 |
| Spleen | 5.7 ± 1.3 | 3.8 ± 0.2 | 4.1 ± 0.5 | 4.4 ± 0.4 |
| Pancreas | 2.4 ± 0.2 | 1.9 ± 0.4 | 1.8 ± 0.5 | 1.6 ± 0.2 |
| Intestine | 2.4 ± 0.6 | 1.6 ± 0.3 | 1.5 ± 0.1 | 1.4 ± 0.2 |
| Kidney | 10.0 ± 1.7 | 6.8 ± 0.8 | 6.5 ± 0.3 | 5.7 ± 0.5 |
| Muscle | 0.7 ± 0.2 | 1.1 ± 0.1 | 1.1 ± 0.1 | 1.1 ± 0.1 |
| Bone | 3.0 ± 0.6 | 1.9 ± 0.2 | 1.9 ± 0.1 | 2.0 ± 0.3 |
| 30 Gy | ||||
| Blood | 43.6 ± 0.9 | 20.9 ± 0.8 | 17.2 ± 1.5 | 13.4 ± 2.5 |
| Lung | 12.8 ± 1.5 | 9.5 ± 0.5 | 6.4 ± 0.7 | 6.5 ± 1.5 |
| Liver | 14.3 ± 1.4 ** | 9.2 ± 0.8 | 7.9 ± 1.0 | 6.3 ± 0.7 * |
| Spleen | 6.3 ± 0.6 | 4.2 ± 0.7 | 4.3 ± 0.6 | 3.9 ± 0.9 |
| Pancreas | 2.4 ± 0.6 | 1.7 ± 0.2 | 1.6 ± 0.2 | 1.3 ± 0.1 |
| Intestine | 2.4 ± 0.3 | 1.4 ± 0.1 | 1.6 ± 0.2 | 1.4 ± 0.2 |
| Kidney | 10.2 ± 0.6 | 6.9 ± 0.4 | 6.3 ± 1.0 | 4.9 ± 0.6 |
| Muscle | 0.6 ± 0.1 | 1.3 ± 0.1 | 1.2 ± 0.2 | 1.1 ± 0.2 |
| Bone | 3.8 ± 0.4 * | 2.6 ± 0.4 * | 2.0 ± 0.4 | 2.0 ± 0.3 |
| Tissue/Organ | 30 Min | Day 1 | Day 2 | Day 4 |
|---|---|---|---|---|
| 0 Gy | ||||
| Blood | 38.9 ± 1.8 | 7.7 ± 0.3 | 6.9 ± 1.2 | 2.4 ± 0.8 |
| Lung | 12.8 ± 1.8 | 3.5 ± 0.3 | 3.2 ± 0.3 | 1.4 ± 0.4 |
| Liver | 8.2 ± 0.6 | 7.3 ± 0.6 | 6.8 ± 0.2 | 4.9 ± 0.9 |
| Spleen | 7.5 ± 0.7 | 9.0 ± 3.8 | 6.6 ± 0.3 | 3.8 ± 1.7 |
| Pancreas | 2.0 ± 0.4 | 0.8 ± 0.1 | 0.8 ± 0.1 | 0.3 ± 0.1 |
| Intestine | 3.7 ± 0.4 | 7.0 ± 1.1 | 5.2 ± 0.5 | 2.3 ± 0.5 |
| Kidney | 8.4 ± 0.4 | 4.2 ± 0.4 | 3.6 ± 0.3 | 2.3 ± 0.4 |
| Muscle | 0.6 ± 0.1 | 0.9 ± 0.0 | 0.9 ± 0.0 | 0.5 ± 0.1 |
| Bone | 4.3 ± 0.8 | 10.8 ± 1.5 | 11.1 ± 0.6 | 6.6 ± 0.7 |
| 30 Gy | ||||
| Blood | 36.1 ± 4.9 | 7.7 ± 0.9 | 5.8 ± 0.8 | 3.5 ± 0.5 |
| Lung | 14.3 ± 2.4 | 3.4 ± 0.7 | 2.6 ± 0.3 | 1.8 ± 0.3 |
| Liver | 8.2 ± 0.6 | 7.8 ± 1.0 | 7.7 ± 0.6 | 7.3 ± 0.4 ** |
| Spleen | 9.2 ± 2.7 | 10.8 ± 3.8 | 9.9 ± 2.6 | 7.2 ± 0.6 |
| Pancreas | 1.9 ± 0.3 | 0.8 ± 0.2 | 0.7 ± 0.1 | 0.5 ± 0.1 |
| Intestine | 3.4 ± 0.7 | 6.6 ± 0.9 | 4.6 ± 1.0 | 3.1 ± 0.2 |
| Kidney | 8.8 ± 1.0 | 3.9 ± 0.4 | 3.6 ± 0.4 | 2.9 ± 0.5 |
| Muscle | 0.8 ± 0.2 | 1.0 ± 0.1 | 1.0 ± 0.3 | 0.6 ± 0.1 |
| Bone | 5.9 ± 1.3 | 11.5 ± 2.3 | 10.6 ± 1.2 | 8.3 ± 0.7 |
| Tissue/Organ | 30 Min | Day 1 | Day 2 | Day 4 |
|---|---|---|---|---|
| 0 Gy | ||||
| Blood | 33.5 ± 1.3 | 16.2 ± 2.1 | 14.7 ± 1.0 | 12.5 ± 0.8 |
| Lung | 13.0 ± 1.3 | 6.9 ± 0.7 | 5.7 ± 1.0 | 5.5 ± 1.1 |
| Liver | 6.3 ± 0.7 | 4.8 ± 0.7 | 4.4 ± 0.5 | 4.8 ± 0.3 |
| Spleen | 4.4 ± 0.5 | 3.2 ± 0.5 | 4.1 ± 0.8 | 3.7 ± 0.7 |
| Pancreas | 2.0 ± 0.5 | 1.5 ± 0.1 | 1.3 ± 0.2 | 1.3 ± 0.2 |
| Intestine | 1.8 ± 0.3 | 1.5 ± 0.2 | 1.4 ± 0.1 | 1.3 ± 0.1 |
| Kidney | 7.5 ± 0.6 | 5.6 ± 0.5 | 6.0 ± 0.3 | 5.0 ± 0.6 |
| Muscle | 0.6 ± 0.1 | 1.0 ± 0.1 | 1.1 ± 0.1 | 0.9 ± 0.1 |
| Bone | 2.7 ± 0.3 | 1.9 ± 0.1 | 2.1 ± 0.5 | 2.6 ± 0.2 |
| 30 Gy | ||||
| Blood | 33.8 ± 1.5 | 18.3 ± 0.7 | 14.8 ± 0.5 | 11.5 ± 1.8 |
| Lung | 12.4 ± 1.8 | 6.3 ± 1.2 | 5.5 ± 0.8 | 5.6 ± 0.7 |
| Liver | 5.7 ± 0.5 | 4.1 ± 0.7 | 3.6 ± 0.4 | 4.5 ± 1.1 |
| Spleen | 4.1 ± 0.8 | 3.7 ± 0.7 | 3.3 ± 0.3 | 3.2 ± 0.4 |
| Pancreas | 1.4 ± 0.2 ** | 1.4 ± 0.2 | 1.4 ± 0.2 | 1.2 ± 0.2 |
| Intestine | 1.9 ± 0.4 | 1.3 ± 0.2 | 1.4 ± 0.2 | 1.2 ± 0.2 |
| Kidney | 7.5 ± 0.6 | 5.3 ± 0.9 | 4.7 ± 0.2 ** | 4.0 ± 0.3 * |
| Muscle | 0.6 ± 0.1 | 1.0 ± 0.1 | 1.0 ± 0.1 | 0.9 ± 0.2 |
| Bone | 2.4 ± 0.1 | 2.2 ± 0.3 | 2.4 ± 0.1 | 2.3 ± 0.3 |
| Tissue/Organ | 30 min | Day 1 | Day 2 | Day 4 |
|---|---|---|---|---|
| 0 Gy | ||||
| Blood | 39.0 ± 1.8 | 17.7 ± 1.4 | 15.4 ± 1.0 | 12.6 ± 1.7 |
| Lung | 13.6 ± 1.5 | 7.9 ± 1.0 | 7.1 ± 0.8 | 5.9 ± 0.5 |
| Liver | 10.2 ± 0.4 | 5.6 ± 0.5 | 5.3 ± 0.8 | 4.9 ± 0.9 |
| Spleen | 10.2 ± 1.1 | 6.6 ± 1.6 | 6.0 ± 0.7 | 4.8 ± 1.1 |
| Pancreas | 2.0 ± 0.4 | 1.8 ± 0.2 | 1.7 ± 0.2 | 1.5 ± 0.3 |
| Intestine | 2.4 ± 0.2 | 2.2 ± 0.3 | 2.0 ± 0.3 | 1.7 ± 0.4 |
| Kidney | 11.1 ± 0.6 | 6.4 ± 1.1 | 6.5 ± 0.7 | 5.1 ± 0.8 |
| Muscle | 0.9 ± 0.2 | 1.4 ± 0.3 | 1.2 ± 0.2 | 1.1 ± 0.1 |
| Bone | 3.8 ± 0.5 | 4.5 ± 0.6 | 4.9 ± 0.9 | 4.5 ± 0.6 |
| 30 Gy | ||||
| Blood | 40.8 ± 2.0 | 19.7 ± 1.2 | 16.8 ± 0.7 | 12.3 ± 1.0 |
| Lung | 15.3 ± 2.6 | 8.4 ± 0.7 | 7.0 ± 0.6 | 6.6 ± 1.9 |
| Liver | 9.3 ± 0.7 * | 6.2 ± 0.1 ** | 5.1 ± 0.5 | 5.1 ± 0.6 |
| Spleen | 13.2 ± 1.8 | 9.3 ± 2.2 | 7.8 ± 0.7 | 5.4 ± 1.3 |
| Pancreas | 1.9 ± 0.6 | 1.7 ± 0.2 | 1.4 ± 0.1 | 1.3 ± 0.1 |
| Intestine | 2.1 ± 0.3 | 2.4 ± 0.2 | 2.1 ± 0.3 | 1.5 ± 0.2 |
| Kidney | 10.1 ± 0.8 | 6.2 ± 1.3 | 5.6 ± 0.4 | 4.9 ± 0.5 |
| Muscle | 0.8 ± 0.2 | 1.3 ± 0.2 | 1.1 ± 0.2 | 1.0 ± 0.1 |
| Bone | 4.0 ± 0.4 | 4.8 ± 0.4 | 4.6 ± 0.6 | 3.7 ± 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugyo, A.; Tsuji, A.B.; Sudo, H.; Takano, K.; Kusakabe, M.; Higashi, T. Proof of Concept Study for Increasing Tenascin-C-Targeted Drug Delivery to Tumors Previously Subjected to Therapy: X-Irradiation Increases Tumor Uptake. Cancers 2020, 12, 3652. https://doi.org/10.3390/cancers12123652
Sugyo A, Tsuji AB, Sudo H, Takano K, Kusakabe M, Higashi T. Proof of Concept Study for Increasing Tenascin-C-Targeted Drug Delivery to Tumors Previously Subjected to Therapy: X-Irradiation Increases Tumor Uptake. Cancers. 2020; 12(12):3652. https://doi.org/10.3390/cancers12123652
Chicago/Turabian StyleSugyo, Aya, Atsushi B. Tsuji, Hitomi Sudo, Kanako Takano, Moriaki Kusakabe, and Tatsuya Higashi. 2020. "Proof of Concept Study for Increasing Tenascin-C-Targeted Drug Delivery to Tumors Previously Subjected to Therapy: X-Irradiation Increases Tumor Uptake" Cancers 12, no. 12: 3652. https://doi.org/10.3390/cancers12123652