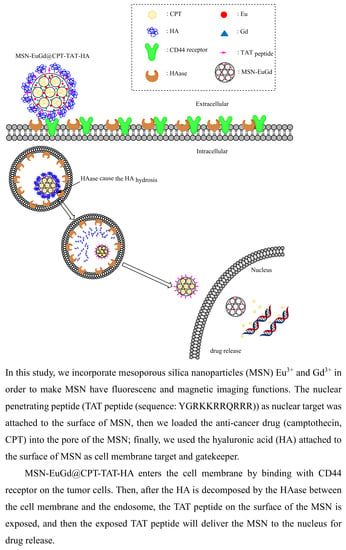
Hyaluronidase-Responsive Mesoporous Silica Nanoparticles with Dual-Imaging and Dual-Target Function
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Eu(NO3)3 and Gd(NO3)3
2.3. Synthesis of MSN-EuGd-NH2
2.4. MSN-EuGd-NH2 loaded into CPT (MSN-EGd-NH2@CPT)
2.5. Synthesis of MSN-EuGd-TAT (or MSN-EuGd@CPT-TAT)
2.6. Synthesis of MSN-EuGd-TAT-HA (or MSN-EuGd@CPT-TAT-HA)
2.7. Characterization
2.8. Drug Release
2.9. In Vitro Experiments
2.9.1. Cell Culture
2.9.2. Cell Viability Assay
2.9.3. Confocal Image Analysis
3. Results and Discussion
3.1. Structure, Formation, Morphology, and Properties of MSNs and EuGd-MSNs
3.2. In Vitro Cytotoxicity and Cellular Uptake of Functionalized MSN-EuGd
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Robert, C.; Wilson, C.S.; Venuta, A.; Ferrari, M.; Arreto, C.D. Evolution of the scientific literature on drug delivery: A 1974-2015 bibliometric study. J. Control. Release 2017, 260, 226–233. [Google Scholar] [CrossRef]
- Szakacs, G.; Paterson, J.K.; Ludwig, J.A.; Booth-Genthe, C.; Gottesman, M.M. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 2006, 5, 219–234. [Google Scholar] [CrossRef]
- Florek, J.; Caillard, R.; Kleitz, F. Evaluation of mesoporous silica nanoparticles for oral drug delivery—Current status and perspective of MSNs drug carriers. Nanoscale 2017, 9, 15252–15277. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Liposomal drug delivery systems: From concept to clinical applications. Adv. Drug Deliv. Rev. 2013, 65, 36–48. [Google Scholar] [CrossRef]
- Soppimath, K.S.; Aminabhavi, T.M.; Kulkarni, A.R.; Rudzinski, W.E. Biodegradable polymeric nanoparticles as drug delivery devices. J. Control. Release 2001, 70, 1–20. [Google Scholar] [CrossRef]
- Jiang, S.L.; Li, M.Y.; Hu, Y.; Zhang, Z.H.; Lv, H.X. Multifunctional self-assembled micelles of galactosamine-hyaluronic acid-vitamin E succinate for targeting delivery of norcantharidin to hepatic carcinoma. Carbohydr. Polym. 2018, 197, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, M.; Zhou, P.; Wu, G.Y.; Wang, L.; Rahoui, N.; Taloub, N.; Huang, X.; Huang, Y.D. Construction of polymer coated core-shell magnetic mesoporous silica nanoparticles with triple responsive drug delivery. Polym. Chem. 2017, 8, 5852–5864. [Google Scholar] [CrossRef]
- Zhao, M.X.; Zhu, B.J. The Research and Applications of Quantum Dots as Nano-Carriers for Targeted Drug Delivery and Cancer Therapy. Nanoscale Res. Lett. 2016, 11, 1–9. [Google Scholar] [CrossRef]
- He, Y.J.; Liang, S.Q.; Long, M.Q.; Xu, H. Mesoporous silica nanoparticles as potential carriers for enhanced drug solubility of paclitaxel. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 12–17. [Google Scholar] [CrossRef]
- Rashidi, L.; Vasheghani-Farahani, E.; Rostami, K.; Ganji, F.; Fallahpour, M. Mesoporous silica nanoparticles with different pore sizes for delivery of pH-sensitive gallic acid. Asia Pac. J. Chem. Eng. 2014, 9, 845–853. [Google Scholar] [CrossRef]
- He, Q.J.; Shi, J.L. Mesoporous silica nanoparticle based nano drug delivery systems: Synthesis, controlled drug release and delivery, pharmacokinetics and biocompatibility. J. Mater. Chem. 2011, 21, 5845–5855. [Google Scholar] [CrossRef]
- Mikada, M.; Sukhbaatar, A.; Miura, Y.; Horie, S.; Sakamoto, M.; Mori, S.; Kodama, T. Evaluation of the enhanced permeability and retention effect in the early stages of lymph node metastasis. Cancer Sci. 2017, 108, 846–852. [Google Scholar] [CrossRef] [Green Version]
- Cui, X.J.; Dong, L.L.; Zhong, S.L.; Shi, C.; Sun, Y.X.; Chen, P. Sonochemical fabrication of folic acid functionalized multistimuli-responsive magnetic graphene oxide-based nanocapsules for targeted drug delivery. Chem. Eng. J. 2017, 326, 839–848. [Google Scholar] [CrossRef]
- Zhang, Q.; Colazo, J.; Berg, D.; Mugo, S.M.; Serpe, M.J. Multiresponsive Nanogels for Targeted Anticancer Drug Delivery. Mol. Pharm. 2017, 14, 2624–2628. [Google Scholar] [CrossRef]
- Li, L.J.; Sun, W.; Li, L.; Liu, Y.Y.; Wu, L.; Wang, F.L.; Zhou, Z.; Zhang, Z.R.; Huang, Y. A pH-responsive sequential-disassembly nanohybrid for mitochondrial targeting. Nanoscale 2017, 9, 314–325. [Google Scholar] [CrossRef]
- Gomes, J.A.P.; Amankwah, R.; Powell-Richards, A.; Dua, H.S. Sodium hyaluronate (hyaluronic acid) promotes migration of human corneal epithelial cells in vitro. Br. J. Ophthalmol. 2004, 88, 821–825. [Google Scholar] [CrossRef] [Green Version]
- Yu, M.H.; Jambhrunkar, S.; Thorn, P.; Chen, J.Z.; Gu, W.Y.; Yu, C.Z. Hyaluronic acid modified mesoporous silica nanoparticles for targeted drug delivery to CD44-overexpressing cancer cells. Nanoscale 2013, 5, 178–183. [Google Scholar] [CrossRef]
- Zhang, M.Z.; Xu, C.L.; Wen, L.Q.; Han, M.K.; Xiao, B.; Zhou, J.; Zhang, Y.C.; Zhang, Z.; Viennois, E.; Merlin, D. A Hyaluronidase-Responsive Nanoparticle-Based Drug Delivery System for Targeting Colon Cancer Cells. Cancer Res. 2016, 76, 7208–7218. [Google Scholar] [CrossRef]
- Pan, L.M.; He, Q.J.; Liu, J.N.; Chen, Y.; Ma, M.; Zhang, L.L.; Shi, J.L. Nuclear-Targeted Drug Delivery of TAT Peptide-Conjugated Monodisperse Mesoporous Silica Nanoparticles. J. Am. Chem. Soc. 2012, 134, 5722–5725. [Google Scholar] [CrossRef]
- Li, Z.H.; Dong, K.; Huang, S.; Ju, E.G.; Liu, Z.; Yin, M.L.; Ren, J.S.; Qu, X.G. A Smart Nanoassembly for Multistage Targeted Drug Delivery and Magnetic Resonance Imaging. Adv. Funct. Mater. 2014, 24, 3612–3620. [Google Scholar] [CrossRef]
- Xiong, L.; Du, X.; Kleitz, F.; Qiao, S.Z. Cancer-Cell-Specific Nuclear-Targeted Drug Delivery by Dual-Ligand-Modified Mesoporous Silica Nanoparticles. Small 2015, 11, 5919–5926. [Google Scholar] [CrossRef]
- Watermann, A.; Brieger, J. Mesoporous Silica Nanoparticles as Drug Delivery Vehicles in Cancer. Nanomaterials 2017, 7, 189. [Google Scholar] [CrossRef]
- Webb, B.A.; Chimenti, M.; Jacobson, M.P.; Barber, D.L. Dysregulated pH: A perfect storm for cancer progression. Nat. Rev. Cancer 2011, 11, 671–677. [Google Scholar] [CrossRef]
- Schafer, F.Q.; Buettner, G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med. 2001, 30, 1191–1212. [Google Scholar] [CrossRef]
- Liu, X.; Shao, W.; Zheng, Y.J.; Yao, C.H.; Peng, L.M.; Zhang, D.M.; Hu, X.Y.; Wang, L.Y. GSH-Responsive supramolecular nanoparticles constructed by beta-D-galactose-modified pillar 5 arene and camptothecin prodrug for targeted anticancer drug delivery. Chem. Commun. 2017, 53, 8596–8599. [Google Scholar] [CrossRef] [PubMed]
- Csoka, A.B.; Scherer, S.W.; Stern, R. Expression analysis of six paralogous human hyaluronidase genes clustered on chromosomes 3p21 and 7q31. Genomics 1999, 60, 356–361. [Google Scholar] [CrossRef]
- Chanmee, T.; Ontong, P.; Itano, N. Hyaluronan: A modulator of the tumor microenvironment. Cancer Lett. 2016, 375, 20–30. [Google Scholar] [CrossRef]
- Khegai, I.I. Neurohormonal Regulation of Tumor Growth. Russ. J. Genet. 2018, 54, 36–44. [Google Scholar] [CrossRef]
- Chan, M.H.; Lin, H.M. Preparation and identification of multifunctional mesoporous silica nanoparticles for in vitro and in vivo dual-mode imaging, theranostics, and targeted tracking. Biomaterials 2015, 46, 149–158. [Google Scholar] [CrossRef]
- Yang, P.P.; Huang, S.S.; Kong, D.Y.; Lin, J.; Fu, H.G. Luminescence functionalization of SBA-15 by YVO4: Eu3+ as a novel drug delivery system. Inorg. Chem. 2007, 46, 3203–3211. [Google Scholar] [CrossRef] [PubMed]
- Damasco, J.A.; Chen, G.Y.; Shao, W.; Agren, H.; Huang, H.Y.; Song, W.T.; Lovell, J.F.; Prasad, P.N. Size-Tunable and Monodisperse Tm3+/Gd3+-Doped Hexagonal NaYbF4 Nanoparticles with Engineered Efficient Near Infrared-to-Near Infrared Upconversion for In Vivo Imaging. ACS Appl. Mater. Interfaces 2014, 6, 13884–13893. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.A.; Selby, L.I.; Johnston, A.P.R.; Such, G.K. The Endosomal Escape of Nanoparticles: Toward More Efficient Cellular Delivery. Bioconjug. Chem. 2019, 30, 263–272. [Google Scholar] [CrossRef]
- Zemtsova, E.G.; Arbenin, A.Y.; Plotnikov, A.F.; Smirnov, V.M. Pore radius fine tuning of a silica matrix (MCM-41) based on the synthesis of alumina nanolayers with different thicknesses by atomic layer deposition. J. Vac. Sci. Technol. A 2015, 33, 6. [Google Scholar] [CrossRef]
- Da Silva, C.R.; Wallau, M.; Urquieta-Gonzalez, E.A. Mesoporous carbons prepared by nano-casting with meso- or non-porous silica nanoparticles. J. Braz. Chem. Soc. 2006, 17, 1170–1180. [Google Scholar] [CrossRef] [Green Version]
- Tao, C.L.; Zhu, Y.F.; Xu, Y.; Zhu, M.; Morita, H.; Hanagata, N. Mesoporous silica nanoparticles for enhancing the delivery efficiency of immunostimulatory DNA drugs. Dalton Trans. 2014, 43, 5142–5150. [Google Scholar] [CrossRef]
- Han, L.; Tang, C.; Yin, C.H. pH-Responsive Core-Shell Structured Nanoparticles for Triple-Stage Targeted Delivery of Doxorubicin to Tumors. ACS Appl. Mater. Interfaces 2016, 8, 23498–23508. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, J. Mesoporous silica nanoparticle-based intelligent drug delivery system for bienzyme-responsive tumour targeting and controlled release. R. Soc. Open Sci. 2018, 5, 1–10. [Google Scholar] [CrossRef]
- O’Donnell, K.P.; Roqan, I.S.; Wang, K.; Lorenz, K.; Alves, E.; Bockowski, M. The photoluminescence/excitation (PL/E) spectroscopy of Eu-implanted GaN. Opt. Mater. 2011, 33, 1063–1065. [Google Scholar] [CrossRef]
- Chen, F.; Huang, P.; Zhu, Y.J.; Wu, J.; Zhang, C.L.; Cui, D.X. The photoluminescence, drug delivery and imaging properties of multifunctional Eu3+/Gd3+ dual-doped hydroxyapatite nanorods. Biomaterials 2011, 32, 9031–9039. [Google Scholar] [CrossRef]
- Ashokan, A.; Menon, D.; Nair, S.; Koyakutty, M. A molecular receptor targeted, hydroxyapatite nanocrystal based multi-modal contrast agent. Biomaterials 2010, 31, 2606–2616. [Google Scholar] [CrossRef]
- Fisichella, M.; Dabboue, H.; Bhattacharyya, S.; Lelong, G.; Saboungi, M.L.; Warmont, F.; Midoux, P.; Pichon, C.; Guerin, M.; Hevor, T.; et al. Uptake of Functionalized Mesoporous Silica Nanoparticles by Human Cancer Cells. J. Nanosci. Nanotechnol. 2010, 10, 2314–2324. [Google Scholar] [CrossRef]
- Antsiferova, Y.; Sotnikova, N.; Parfenyuk, E. Different Effects of the Immunomodulatory Drug GMDP Immobilized onto Aminopropyl Modified and Unmodified Mesoporous Silica Nanoparticles upon Peritoneal Macrophages of Women with Endometriosis. Biomed Res. Int. 2013, 2013, 924362. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.P.; Zhang, L.; Zhu, Y.; Shen, X.C.; Ji, S.C.; Tan, X.Y.; Cheng, L.; Liang, H. Water-soluble hyaluronic acid-hybridized polyaniline nanoparticles for effectively targeted photothermal therapy. J. Mater. Chem. B 2015, 3, 3767–3776. [Google Scholar] [CrossRef]
- Chou, C.C.; Chen, W.; Hung, Y.; Mou, C.Y. Molecular Elucidation of Biological Response to Mesoporous Silica Nanoparticles in Vitro and in Vivo. ACS Appl. Mater. Interfaces 2017, 9, 22235–22251. [Google Scholar] [CrossRef]







| Physical Data | MSN | MSN-EuGd |
|---|---|---|
| Brunauer–Emmett–Teller (BET) Surface Area (m2/g) | 947.57 | 608.19 |
| Pore Volume (cm3/g) | 0.77 | 0.93 |
| Barrett–Joyner–Halenda (BJH) Desorption Diameter (nm) | 2.29 | 2.75 |
| X-ray diffraction (XRD) 2θ (°) | 2.40 | 2.21 |
| d100-spacing (nm) | 3.68 | 3.99 |
| Wall thickness (nm) | 1.95 | 1.86 |
| Mean particle diameters (nm) | 197 | 271 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.-Y.; Lee, C.-C.; Lin, H.-M. Hyaluronidase-Responsive Mesoporous Silica Nanoparticles with Dual-Imaging and Dual-Target Function. Cancers 2019, 11, 697. https://doi.org/10.3390/cancers11050697
Wu Z-Y, Lee C-C, Lin H-M. Hyaluronidase-Responsive Mesoporous Silica Nanoparticles with Dual-Imaging and Dual-Target Function. Cancers. 2019; 11(5):697. https://doi.org/10.3390/cancers11050697
Chicago/Turabian StyleWu, Zhi-Yuan, Cheng-Chang Lee, and Hsiu-Mei Lin. 2019. "Hyaluronidase-Responsive Mesoporous Silica Nanoparticles with Dual-Imaging and Dual-Target Function" Cancers 11, no. 5: 697. https://doi.org/10.3390/cancers11050697