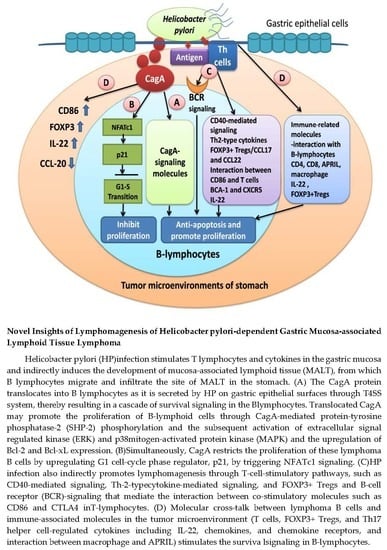
Novel Insights of Lymphomagenesis of Helicobacter pylori-Dependent Gastric Mucosa-Associated Lymphoid Tissue Lymphoma
Abstract
:1. Introduction
2. Indirect Interaction Between Tumor-Infiltrating T Cells and B-Cell lymphoma of Gastric MALT Lymphoma
3. Chemokines and Their Receptors, and Regulatory T-Cells in Gastric MALT Lymphoma
4. Epigenetic Changes and Genetic Changes Are Involved in the Lymphomagenesis of Gastric MALT Lymphoma
5. The Direct Evidence of Cytotoxin-Associated Gene A in the Lymphomagenesis of Gastric MALT Lymphoma
6. CagA and Its Regulated Immune Response in Gastric Microenvironment May Participate in the Lymphomagenesis of Gastric MALT Lymphoma
7. Microbiomes May Be Associated with Antibiotic-Responsiveness of Gastric MALT Lymphoma
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Du, M.Q.; Isaccson, P.G. Gastric MALT lymphoma: From aetiology to treatment. Lancet Oncol. 2002, 3, 97–104. [Google Scholar] [CrossRef]
- Isaacson, P.G.; Du, M.Q. Gastrointestinal lymphoma: Where morphology meets molecular biology. J. Pathol. 2005, 205, 255–274. [Google Scholar] [CrossRef] [PubMed]
- Doglioni, C.; Ponzoni, M.; Ferreri, A.J.; Savio, A.; Gruppo Italiano Patologi Apparato Digerente (GIPAD); Società Italiana di Anatomia Patologica e Citopatologia Diagnostica/International Academy of Pathology, Italian division (SIAPEC/IAP). Gastric lymphoma: The histology report. Dig. Liver Dis. 2011, 43 (Suppl. 4), S310–S318. [Google Scholar] [CrossRef]
- Kuo, S.H.; Cheng, A.L. Helicobacter pylori and mucosa-associated lymphoid tissue: What’s new. Hematol. Am. Soc. Hematol. Educ. Program. 2013, 2013, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Zullo, A.; Hassan, C.; Ridola, L.; Repici, A.; Manta, R.; Andriani, A. Gastric MALT lymphoma: Old and new insights. Ann. Gastroenterol. 2014, 27, 27–33. [Google Scholar]
- Jaffe, E.S.; Harris, N.L.; Stein, H.; Isaacson, P.G. Classification of lymphoid neoplasms: The microscope as a tool for disease discovery. Blood 2008, 112, 4384–4399. [Google Scholar] [CrossRef]
- Sagaert, X.; Tousseyn, T.; Yantiss, R.K. Gastrointestinal B-cell lymphomas: From understanding B-cell physiology to classification and molecular pathology. World J. Gastrointest. Oncol. 2012, 4, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, A.L.; Rosenwald, A.; Staudt, L.M. Lymphoid malignancies: The dark side of B-cell differentiation. Nat. Rev. Immunol. 2002, 2, 920–932. [Google Scholar] [CrossRef]
- Sagaert, X.; Sprangers, B.; De Wolf-Peeters, C. The dynamics of the B follicle: Understanding the normal counterpart of B-cell-derived malignancies. Leukemia 2007, 21, 1378–1386. [Google Scholar] [CrossRef]
- Bautista-Quach, M.A.; Ake, C.D.; Chen, M.; Wang, J. Gastrointestinal lymphomas: Morphology, immunophenotype and molecular features. J. Gastrointest. Oncol. 2012, 3, 209–225. [Google Scholar]
- Jaso, J.; Chen, L.; Li, S.; Lin, P.; Chen, W.; Miranda, R.N.; Konoplev, S.; Medeiros, L.J.; Yin, C.C. CD5-positive mucosa-associated lymphoid tissue (MALT) lymphoma: A clinicopathologic study of 14 cases. Hum. Pathol. 2012, 43, 1436–1443. [Google Scholar] [CrossRef]
- Juárez-Salcedo, L.M.; Sokol, L.; Chavez, J.C.; Dalia, S. Primary Gastric Lymphoma, Epidemiology, Clinical Diagnosis, and Treatment. Cancer Control 2018, 25, 1073274818778256. [Google Scholar] [CrossRef]
- Wotherspoon, A.C.; Ortiz-Hidalgo, C.; Falzon, M.R.; Isaacson, P.G. Helicobacter pylori associated gastritis and primary B-cell gastric lymphoma. Lancet 1991, 338, 1175–1176. [Google Scholar] [CrossRef]
- Wotherspoon, A.C.; Doglioni, C.; Diss, T.C.; Pan, L.; Moschini, A.; de Boni, M.; Isaacson, P.G. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet 1993, 342, 575–577. [Google Scholar] [CrossRef]
- Zullo, A.; Hassan, C.; Andriani, A.; Cristofari, F.; De Francesco, V.; Ierardi, E.; Tomao, S.; Morini, S.; Vaira, D. Eradication therapy for Helicobacter pylori in patients with gastric MALT lymphoma: A pooled data analysis. Am. J. Gastroenterol. 2009, 104, 1932–1937; quiz 1938. [Google Scholar] [CrossRef] [PubMed]
- Zullo, A.; Hassan, C.; Cristofari, F.; Andriani, A.; De Francesco, V.; Ierardi, E.; Tomao, S.; Stolte, M.; Morini, S.; Vaira, D. Effects of Helicobacter pylori eradication on early stage gastric mucosa-associated lymphoid tissue lymphoma. Clin. Gastroenterol. Hepatol. 2010, 8, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Kuo, S.H.; Chen, L.T.; Lin, C.W.; Wu, M.S.; Hsu, P.N.; Tsai, H.J.; Chu, C.Y.; Tzeng, Y.S.; Wang, H.P.; Yeh, K.H.; et al. Detection of the Helicobacter pylori CagA protein in gastric mucosa-associated lymphoid tissue lymphoma cells: Clinical and biological significance. Blood Cancer J. 2013, 3, e125. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Chung, S.J.; Choi, Y.S.; Cheon, J.H.; Kim, C.W.; Kim, S.G.; Jung, H.C.; Song, I.S. Helicobacter pylori eradication for low-grade gastric mucosa-associated lymphoid tissue lymphoma is more successful in inducing remission in distal compared to proximal disease. Br. J. Cancer 2007, 96, 1324–1328. [Google Scholar] [CrossRef] [PubMed]
- Wotherspoon, A.C. Gastric lymphoma of mucosa-associated lymphoid tissue and Helicobacter pylori. Annu. Rev. Med. 1998, 49, 289–299. [Google Scholar] [CrossRef]
- Alzahrani, S.; Lina, T.T.; Gonzalez, J.; Pinchuk, I.V.; Beswick, E.J.; Reyes, V.E. Effect of Helicobacter pylori on gastric epithelial cells. World J. Gastroenterol. 2014, 20, 12767–12780. [Google Scholar] [CrossRef]
- Zullo, A.; Hassan, C.; Ridola, L.; De Francesco, V.; Rossi, L.; Tomao, S.; Vaira, D.; Genta, R.M. Eradication therapy in Helicobacter pylori-negative, gastric low-grade mucosa-associated lymphoid tissue lymphoma patients: A systematic review. J. Clin. Gastroenterol. 2013, 47, 824–827. [Google Scholar] [CrossRef] [PubMed]
- Asano, N.; Iijima, K.; Koike, T.; Imatani, A.; Shimosegawa, T. Helicobacter pylori-negative gastric mucosa-associated lymphoid tissue lymphomas: A review. World J. Gastroenterol. 2015, 21, 8014–8020. [Google Scholar] [CrossRef] [PubMed]
- Raderer, M.; Wöhrer, S.; Kiesewetter, B.; Dolak, W.; Lagler, H.; Wotherspoon, A.; Muellauer, L.; Chott, A. Antibiotic treatment as sole management of Helicobacter pylori-negative gastric MALT lymphoma: A single center experience with prolonged follow-up. Ann. Hematol. 2015, 94, 969–973. [Google Scholar] [CrossRef]
- Kuo, S.H.; Yeh, K.H.; Wu, M.S.; Lin, C.W.; Wei, M.F.; Liou, J.M.; Wang, H.P.; Chen, L.T.; Cheng, A.L. First-line antibiotic therapy in Helicobacter pylori-negative low-grade gastric mucosa-associated lymphoid tissue lymphoma. Sci. Rep. 2017, 7, 14333. [Google Scholar] [CrossRef] [PubMed]
- Farinha, P.; Gascoyne, R.D. Helicobacter pylori and MALT lymphoma. Gastroenterology 2005, 128, 1579–1605. [Google Scholar] [CrossRef]
- Du, M.Q. MALT lymphoma: Many roads lead to nuclear factor-kb activation. Histopathology 2011, 58, 26–38. [Google Scholar] [CrossRef]
- Lin, W.C.; Tsai, H.F.; Kuo, S.H.; Wu, M.S.; Lin, C.W.; Hsu, P.I.; Cheng, A.L.; Hsu, P.N. Translocation of Helicobacter pylori CagA into Human B lymphocytes, the origin of mucosa-associated lymphoid tissue lymphoma. Cancer Res. 2010, 70, 5740–5748. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, N.; Yuasa, H.; Tanaka, S.; Sawa, H.; Miura, M.; Matsui, A.; Higashi, H.; Musashi, M.; Iwabuchi, K.; Suzuki, M.; et al. Transgenic expression of Helicobacter pylori CagA induces gastrointestinal and hematopoietic neoplasms in mouse. Proc. Natl. Acad. Sci. USA 2008, 105, 1003–1008. [Google Scholar] [CrossRef]
- Greiner, A.; Marx, A.; Heesemann, J.; Leebmann, J.; Schmausser, B.; Muller-Hermelink, H.K. Idiotype identity in a MALT type lymphoma and B cells in Helicobacter pylori associated chronic gastritis. Lab. Investig. 1994, 70, 572–578. [Google Scholar]
- Hussell, T.; Isaacson, P.G.; Crabtree, J.E.; Spencer, J. Helicobacter pylori-specific tumour-infiltrating T cells provide contact dependent help for the growth of malignant B cells in low-grade gastric lymphoma of mucosa-associated lymphoid tissue. J. Pathol. 1996, 178, 122–127. [Google Scholar] [CrossRef]
- Hauer, A.C.; Finn, T.M.; MacDonald, T.T.; Spencer, J.; Isaacson, P.G. Analysis of TH1 and TH2 cytokine production in low-grade B-cell gastric MALT-type lymphomas stimulated in vitro with Helicobacter pylori. J. Clin. Pathol. 1997, 50, 957–959. [Google Scholar] [CrossRef] [PubMed]
- Greiner, A.; Knorr, C.; Qin, Y.; Sebald, W.; Schimpl, A.; Banchereau, J.; Müller-Hermelink, H.K. Low-grade B-cell lymphomas of mucosa-associated lymphoid tissue (MALT-type) require CD40-mediated signalling and Th2-type cytokines for in vitro growth and differentiation. Am. J. Pathol. 1997, 150, 1583–1593. [Google Scholar] [PubMed]
- Mueller, A.; O’rouuke, J.; Chu, P.; Chu, A.; Dixon, M.F.; Bouley, D.M.; Lee, A.; Falkow, S. The role of antigenic drive and tumor-infiltrating accessory cells in the pathogenesis of Helicobacter-induced mucosa-associated lymphoid tissue lymphoma. Am. J. Pathol. 2005, 167, 797–812. [Google Scholar] [CrossRef]
- D’Elios, M.M.; Amedei, A.; Manghetti, M.; Costa, F.; Baldari, C.T.; Quazi, A.S.; Telford, J.L.; Romagnani, S.; Del Prete, G. Impaired T-cell regulation of B-cell growth in Helicobacter pylori-related gastric low-grade MALT lymphoma. Gastroenterology 1999, 117, 1105–1112. [Google Scholar] [CrossRef]
- Bergman, M.P.; D’Elios, M.M. Cytotoxic T cells in H. pylori-related gastric autoimmunity and gastric lymphoma. J. Biomed. Biotechnol. 2010, 2010, 104918. [Google Scholar] [CrossRef] [PubMed]
- Vyth-Dreese, F.A.; Boot, H.; Dellemijn, T.A.; Majoor, D.M.; Oomen, L.C.; Laman, J.D.; Van Meurs, M.; De Weger, R.A.; De Jong, D. Localization in situ of costimulatory molecules and cytokines in B-cell non-Hodgkin’s lymphoma. Immunology 1998, 94, 580–586. [Google Scholar] [CrossRef]
- Lane, P. Regulation of T and B cell responses by modulating interactions between CD28/CTLA4 and their ligands, CD80 and CD86. Ann. N. Y. Acad. Sci. 1997, 815, 392–400. [Google Scholar] [CrossRef]
- Ikemizu, S.; Gilbert, R.J.; Fennelly, J.A.; Collins, A.V.; Harlos, K.; Jones, E.Y.; Stuart, D.I.; Davis, S.J. Structure and dimerization of a soluble form of B7-1. Immunity 2000, 12, 51–60. [Google Scholar] [CrossRef]
- Suvas, S.; Singh, V.; Sahdev, S.; Vohra, H.; Agrewala, J.N. Distinct role of CD80 and CD86 in the regulation of the activation of B cell and B cell lymphoma. J. Biol. Chem. 2002, 277, 7766–7775. [Google Scholar] [CrossRef]
- De Jong, D.; Vyth-Dreese, F.; Dellemijn, T.; Verra, N.; Ruskoné-Fourmestraux, A.; Lavergne-Slove, A.; Hart, G.; Boot, H. Histological and immunological parameters to predict treatment outcome of Helicobacter pylori eradication in low-grade gastric MALT lymphoma. J. Pathol. 2001, 193, 318–324. [Google Scholar] [CrossRef]
- Du, M.Q. MALT lymphoma: Genetic abnormalities, immunological stimulation and molecular mechanism. Best Pract. Res. Clin. Haematol. 2017, 30, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Hussell, T.; Isaacson, P.G.; Spencer, J. Proliferation and differentiation of tumour cells from B-cell lymphoma of mucosa-associated lymphoid tissue in vitro. J. Pathol. 1993, 169, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Craig, V.J.; Arnold, I.; Gerke, C.; Huynh, M.Q.; Wundisch, T.; Neubauer, A.; Renner, C.; Falkow, S.; Müller, A. Gastric MALT lymphoma B cells express polyreactive, somatically mutated immunoglobulins. Blood 2010, 115, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Bende, R.J.; Aarts, W.M.; Riedl, R.G.; de Jong, D.; Pals, S.T.; van Noesel, C.J. Among B cell non-Hodgkin’s lymphomas, MALT lymphomas express a unique antibody repertoire with frequent rheumatoid factor reactivity. J. Exp. Med. 2005, 201, 1229–1241. [Google Scholar] [CrossRef] [PubMed]
- Dagklis, A.; Ponzoni, M.; Govi, S.; Cangi, M.G.; Pasini, E.; Charlotte, F.; Vino, A.; Doglioni, C.; Davì, F.; Lossos, I.S.; et al. Immunoglobulin gene repertoire in ocular adnexal lymphomas: Hints on the nature of the antigenic stimulation. Leukemia 2012, 26, 814–821. [Google Scholar] [CrossRef]
- Michaeli, M.; Tabibian-Keissar, H.; Schiby, G.; Shahaf, G.; Pickman, Y.; Hazanov, L.; Rosenblatt, K.; Dunn-Walters, D.K.; Barshack, I.; Mehr, R. Immunoglobulin gene repertoire diversification and selection in the stomach—From gastritis to gastric lymphomas. Front. Immunol. 2014, 5, 264. [Google Scholar] [CrossRef] [PubMed]
- Silverman, G.J.; Goni, F.; Fernandez, J.; Chen, P.P.; Frangione, B.; Carson, D.A. Distinct patterns of heavy chain variable region subgroup use by human monoclonal autoantibodies of different specificity. J. Exp. Med. 1988, 168, 2361–2366. [Google Scholar] [CrossRef]
- Ramsland, P.A.; Guddat, L.W.; Edmundson, A.B.; Raison, R.L. Diverse binding site structures revealed in homology models of polyreactive immunoglobulins. J. Comput. Aided Mol. Des. 1997, 11, 453–461. [Google Scholar] [CrossRef]
- Mazzucchelli, L.; Blaser, A.; Kappeler, A.; Schärli, P.; Laissue, J.A.; Baggiolini, M.; Uguccioni, M. BCA-1 is highly expressed in Helicobacter pylori-induced mucosa-associated lymphoid tissue and gastric lymphoma. J. Clin. Investig. 1999, 104, R49–R54. [Google Scholar] [CrossRef]
- Jones, D.; Benjamin, R.J.; Shahsafaei, A.; Dorfman, D.M. The chemokine receptor CXCR3 is expressed in a subset of B-cell lymphomas and is a marker of B-cell chronic lymphocytic leukemia. Blood 2000, 95, 627–632. [Google Scholar]
- Ohshima, K.; Suefuji, H.; Karube, K.; Hamasaki, M.; Hatano, B.; Tutiya, T.; Yamaguchi, T.; Suzuki, K.; Suzumiya, J.; Kikuchi, M. Expression of chemokine receptor CXCR3 and its ligand, mig, in gastric and thyroid marginal zone lymphomas. Possible migration and autocrine mechanism. Leuk. Lymphoma 2003, 44, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Suefuji, H.; Ohshima, K.; Karube, K.; Kawano, R.; Nabeshima, K.; Suzumiya, J.; Hayabuchi, N.; Kikuchi, M. CXCR3-positive B cells found at elevated frequency in the peripheral blood of patients with MALT lymphoma are attracted by MIG and belong to the lymphoma clone. Int. J. Cancer 2005, 114, 896–901. [Google Scholar] [CrossRef]
- Yamamoto, H.; Nakamura, T.; Matsuo, K.; Tajika, M.; Kawai, H.; Ohmiya, N.; Niwa, Y.; Goto, H.; Nakamura, S. Significance of CXCR3 expression in gastric low-grade B-cell lymphoma of mucosa-associated lymphoid tissue type for predicting responsiveness to Helicobacter pylori eradication. Cancer Sci. 2008, 99, 1769–1773. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, A.J.; Steinbauer, E.; Hofmann, N.A.; Strunk, D.; Gerlza, T.; Beham-Schmid, C.; Schaider, H.; Neumeister, P. Chemokine receptors in gastric MALT lymphoma: Loss of CXCR4 and upregulation of CXCR7 is associated with progression to diffuse large B-cell lymphoma. Mod. Pathol. 2013, 26, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Stollberg, S.; Kämmerer, D.; Neubauer, E.; Schulz, S.; Simonitsch-Klupp, I.; Kiesewetter, B.; Raderer, M.; Lupp, A. Differential somatostatin and CXCR4 chemokine receptor expression in MALT-type lymphoma of gastric and extragastric origin. J. Cancer Res. Clin. Oncol. 2016, 142, 2239–2247. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Beaty, N.; Chen, S.; Qi, C.F.; Masiuk, M.; Shin, D.M.; Morse, H.C., 3rd. The CXCR7 chemokine receptor promotes B-cell retention in the splenic marginal zone and serves as a sink for CXCL12. Blood 2012, 119, 465–468. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, N.; Azadegan-Dehkordi, F.; Rahimian, G.; Rafieian-Kopaei, M.; Shirzad, H. Role of Regulatory T-cells in Different Clinical Expressions of Helicobacter pylori Infection. Arch. Med. Res. 2016, 47, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, A.; Strömberg, E.; Sjöling, A.; Lindholm, C.; Enarsson, K.; Edebo, A.; Johnsson, E.; Suri-Payer, E.; Larsson, P.; Rudin, A.; et al. Mucosal FOXP3-expressing CD4+CD25high regulatory T cells in Helicobacter pylori-infected patients. Infect. Immun. 2005, 73, 523–531. [Google Scholar] [CrossRef]
- Rad, R.; Brenner, L.; Bauer, S.; Schwendy, S.; Layland, L.; da Costa, C.P.; Reindl, W.; Dossumbekova, A.; Friedrich, M.; Saur, D.; et al. CD25+/Foxp3+ T cells regulate gastric inflammation and Helicobacter pylori colonization in vivo. Gastroenterology 2006, 131, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Fontenot, J.D.; Gavin, M.A.; Rudensky, A.Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003, 4, 330–336. [Google Scholar] [CrossRef]
- Yang, Z.Z.; Novak, A.J.; Stenson, M.J.; Witzig, T.E.; Ansell, S.M. Intratumoral CD4+CD25+ regulatory T-cell-mediated suppression of infiltrating CD4+ T-cells in B-cell non-Hodgkin lymphoma. Blood 2006, 107, 3639–3646. [Google Scholar] [CrossRef] [PubMed]
- Craig, V.J.; Cogliatti, S.B.; Arnold, I.; Gerke, C.; Balandat, J.E.; Wündisch, T.; Müller, A. B-cell receptor signaling and CD40 ligand-independent T cell help cooperate in Helicobacter-induced MALT lymphomagenesis. Leukemia 2010, 24, 1186–1196. [Google Scholar] [CrossRef] [PubMed]
- García, M.; Bellosillo, B.; Sánchez-González, B.; García-Payarols, F.; Seoane, A.; Ferrer, A.M.; Gimeno, E.; Barranco, L.E.; Torner, A.; Solé, F.; et al. Study of regulatory T-cells in patients with gastric malt lymphoma: Influence on treatment response and outcome. PLoS ONE 2012, 7, e51681. [Google Scholar] [CrossRef] [PubMed]
- Iwaya, Y.; Kobayashi, M.; Momose, M.; Hiraoka, N.; Sakai, Y.; Akamatsu, T.; Tanaka, E.; Ohtani, H.; Fukuda, M.; Nakayama, J. High levels of FOXP3+ regulatory T cells in gastric MALT lymphoma predict responsiveness to Helicobacter pylori eradication. Helicobacter 2013, 18, 356–362. [Google Scholar] [CrossRef]
- Martín-Subero, J.I.; Kreuz, M.; Bibikova, M.; Bentink, S.; Ammerpohl, O.; Wickham-Garcia, E.; Rosolowski, M.; Richter, J.; Lopez-Serra, L.; Ballestar, E.; et al. New insights into the biology and origin of mature aggressive B-cell lymphomas by combined epigenomic, genomic, and transcriptional profiling. Blood 2009, 113, 2488–2497. [Google Scholar] [CrossRef]
- Oka, T.; Ouchida, M.; Koyama, M.; Ogama, Y.; Takada, S.; Nakatani, Y.; Tanaka, T.; Yoshino, T.; Hayashi, K.; Ohara, N.; et al. Gene silencing of the tyrosine phosphatase SHP1 gene by aberrant methylation in leukemias/lymphomas. Cancer Res. 2002, 62, 6390–6394. [Google Scholar] [PubMed]
- Koyama, M.; Oka, T.; Ouchida, M.; Nakatani, Y.; Nishiuchi, R.; Yoshino, T.; Hayashi, K.; Akagi, T.; Seino, Y. Activated proliferation of B-cell lymphomas/leukemias with the SHP1 gene silencing by aberrant CpG methylation. Lab. Investig. 2003, 83, 1849–1858. [Google Scholar] [CrossRef]
- Jones, P.A.; Takai, D. The role of DNA methylation in mammalian epigenetics. Science 2001, 293, 1068–1070. [Google Scholar] [CrossRef]
- Perri, F.; Cotugno, R.; Piepoli, A.; Merla, A.; Quitadamo, M.; Gentile, A.; Pilotto, A.; Annese, V.; Andriulli, A. Aberrant DNA methylation in non-neoplastic gastric mucosa of H. pylori infected patients and effect of eradication. Am. J. Gastroenterol. 2007, 102, 1361–1371. [Google Scholar] [CrossRef]
- Ando, T.; Yoshida, T.; Enomoto, S.; Asada, K.; Tatematsu, M.; Ichinose, M.; Sugiyama, T.; Ushijima, T. DNA methylation of microRNA genes in gastric mucosae of gastric cancer patients: Its possible involvement in the formation of epigenetic field defect. Int. J. Cancer 2009, 124, 2367–2374. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, J.S.; Jung, H.C.; Lee, C.H.; Kim, C.W.; Song, I.S.; Kim, C.Y. Regression of low-grade gastric mucosa-associated lymphoid tissue lymphoma after eradication of Helicobacter pylori: Possible association with p16 hypermethylation. J. Gastroenterol. 2002, 37, 17–22. [Google Scholar] [CrossRef]
- Min, K.O.; Seo, E.J.; Kwon, H.J.; Lee, E.J.; Kim, W.I.; Kang, C.S.; Kim, K.M. Methylation of p16(INK4A) and p57(KIP2) are involved in the development and progression of gastric MALT lymphomas. Mod. Pathol. 2006, 19, 141–148. [Google Scholar] [CrossRef]
- Park, S.; Kim, K.M.; Kim, J.J.; Lee, J.H.; Rhee, J.C.; Ko, Y.H. Methylation of p16INK4A and mitotic arrest defective protein 2 (MAD2) genes in gastric marginal-zone B-cell lymphomas. Acta Haematol. 2008, 120, 217–224. [Google Scholar] [CrossRef]
- Kaneko, Y.; Sakurai, S.; Hironaka, M.; Sato, S.; Oguni, S.; Sakuma, Y.; Sato, K.; Sugano, K.; Saito, K. Distinct methylated profiles in Helicobacter pylori dependent and independent gastric MALT lymphomas. Gut 2003, 52, 641–646. [Google Scholar] [CrossRef]
- Kondo, T.; Oka, T.; Sato, H.; Shinnou, Y.; Washio, K.; Takano, M.; Morito, T.; Takata, K.; Ohara, N.; Ouchida, M.; et al. Accumulation of aberrant CpG hypermethylation by Helicobacter pylori infection promotes development and progression of gastric MALT lymphoma. Int. J. Oncol. 2009, 35, 547–557. [Google Scholar]
- Muhammad, J.S.; Eladl, M.A.; Khoder, G. Helicobacter pylori-induced DNA Methylation as an Epigenetic Modulator of Gastric Cancer: Recent Outcomes and Future Direction. Pathogens 2019, 8, 23. [Google Scholar] [CrossRef]
- Houghton, J.; Fox, J.G.; Wang, T.C. Gastric cancer: Laboratory bench to clinic. J. Gastroenterol. Hepatol. 2002, 17, 495–502. [Google Scholar] [CrossRef]
- Wu, M.S.; Chen, L.T.; Shun, C.T.; Huang, S.P.; Chiu, H.M.; Wang, H.P.; Lin, M.T.; Cheng, A.L.; Lin, J.T. Promoter polymorphisms of tumor necrosis factor-alpha are associated with risk of gastric mucosa-associated lymphoid tissue lymphoma. Int. J. Cancer 2004, 110, 695–700. [Google Scholar] [CrossRef]
- Wu, M.S.; Shun, C.T.; Huang, S.P.; Cheng, A.L.; Chenm, L.T.; Lin, J.T. Effect of interleukin-1beta and glutathione S-transferase genotypes on the development of gastric mucosa-associated lymphoid tissue lymphoma. Haematologica 2004, 89, 1015–1017. [Google Scholar]
- Cheng, T.Y.; Lin, J.T.; Chen, L.T.; Shun, C.T.; Wang, H.P.; Lin, M.T.; Wang, T.E.; Cheng, A.L.; Wu, M.S. Association of T-cell regulatory gene polymorphisms with susceptibility to gastric mucosa-associated lymphoid tissue lymphoma. J. Clin. Oncol. 2006, 24, 3483–3489. [Google Scholar] [CrossRef]
- Liao, F.; Hsu, Y.C.; Kuo, S.H.; Yang, Y.C.; Chen, J.P.; Hsu, P.N.; Lin, C.W.; Chen, L.T.; Cheng, A.L.; Fann, C.S.; et al. Genetic polymorphisms and tissue expression of interleukin-22 associated with risk and therapeutic response of gastric mucosa-associated lymphoid tissue lymphoma. Blood Cancer J. 2014, 4, eXX. [Google Scholar] [CrossRef]
- Chen, J.P.; Wu, M.S.; Kuo, S.H.; Liao, F. IL-22 negatively regulates Helicobacter pylori-induced CCL20 expression in gastric epithelial cells. PLoS ONE 2014, 9, e97350. [Google Scholar] [CrossRef]
- Stein, M.; Bagnoli, F.; Halenbeck, R.; Rappuoli, R.; Fantl, W.J.; Covacci, A. c-Src/Lyn kinases activate Helicobacter pylori CagA through tyrosine phosphorylation of the EPIYA motifs. Mol. Microbiol. 2002, 43, 971–980. [Google Scholar] [CrossRef]
- Hatakeyama, M. Oncogenic mechanisms of the Helicobacter pylori CagA protein. Nat. Rev. Cancer 2004, 4, 688–694. [Google Scholar] [CrossRef]
- Stein, M.; Ruggiero, P.; Rappuoli, R.; Bagnoli, F. Helicobacter pylori CagA: From pathogenic mechanisms to its use as an anti-cancer vaccine. Front. Immunol. 2013, 4, 328. [Google Scholar] [CrossRef]
- Ghoshal, U.C.; Tiwari, S.; Dhingra, S.; Pandey, R.; Ghoshal, U.; Tripathi, S.; Singh, H.; Gupta, V.K.; Nagpal, A.K.; Naik, S.; et al. Frequency of Helicobacter pylori and CagA antibody in patients with gastric neoplasms and controls: The Indian enigma. Dig. Dis. Sci. 2008, 53, 1215–1222. [Google Scholar] [CrossRef]
- Achyut, B.R.; Moorchung, N.; Srivastava, A.N.; Gupta, N.K.; Mittal, B. Risk of lymphoid follicle development in patients with chronic antral gastritis: Role of endoscopic features, histopathological parameters, CagA status and interleukin-1 gene polymorphisms. Inflamm. Res. 2008, 57, 51–55. [Google Scholar] [CrossRef]
- Eck, M.; Schmausser, B.; Haas, R.; Greiner, A.; Czub, S.; Müller-Hermelink, H.K. MALT-type lymphoma of the stomach is associated with Helicobacter pylori strains expressing the CagA protein. Gastroenterology 1997, 112, 1482–1486. [Google Scholar] [CrossRef]
- Sumida, T.; Kitadai, Y.; Hiyama, T.; Shinagawa, K.; Tanaka, M.; Kodama, M.; Masuda, H.; Ito, M.; Tanaka, S.; Yoshihara, M.; et al. Antibodies to Helicobacter pylori and CagA protein are associated with the response to antibacterial therapy in patients with H. pylori-positive API2-MALT1-negative gastric MALT lymphoma. Cancer Sci. 2009, 100, 1075–1081. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, C.; Huang, J.; Ge, Z.; Dong, Q.; Zhong, X.; Su, Y.; Zheng, S. The Helicobacter pylori virulence factor CagA promotes Erk1/2-mediated Bad phosphorylation in lymphocytes: A mechanism of CagA-inhibited lymphocyte apoptosis. Cell. Microbiol. 2007, 9, 952–961. [Google Scholar] [CrossRef]
- Kuo, S.H.; Yeh, K.H.; Chen, L.T.; Lin, C.W.; Hsu, P.N.; Wu, M.S.; Liou, J.M.; Tsai, H.J.; Tzeng, Y.S.; Cheng, A.L. Helicobacter pylori CagA Translocation Is Closely Associated with the Expression of CagA-signaling Molecules in Low-grade Gastric Mucosa-associated Lymphoid Tissue Lymphoma. Am. J. Surg. Pathol. 2015, 39, 761–766. [Google Scholar] [CrossRef]
- Umehara, S.; Higashi, H.; Ohnishi, N.; Asaka, M.; Hatakeyama, M. Effects of Helicobacter pylori CagA protein on the growth and survival of B lymphocytes, the origin of MALT lymphoma. Oncogene 2003, 22, 8337–8342. [Google Scholar] [CrossRef]
- Kido, M.; Watanabe, N.; Aoki, N.; Iwamoto, S.; Nishiura, H.; Maruoka, R.; Ikeda, A.; Azuma, T.; Chiba, T. Dual roles of CagA protein in Helicobacterpylori-induced chronic gastritis in mice. Biochem. Biophys. Res. Commun. 2011, 412, 266–272. [Google Scholar] [CrossRef]
- Bagheri, N.; Shirzad, H.; Elahi, S.; Azadegan-Dehkordi, F.; Rahimian, G.; Shafigh, M.; Rashidii, R.; Sarafnejad, A.; Rafieian-Kopaei, M.; Faridani, R.; et al. Downregulated regulatory T cell function is associated with increased peptic ulcer in Helicobacter pylori-infection. Microb. Pathog. 2017, 110, 165–175. [Google Scholar] [CrossRef]
- Lina, T.T.; Alzahrani, S.; House, J.; Yamaoka, Y.; Sharpe, A.H.; Rampy, B.A.; Pinchuk, I.V.; Reyes, V.E. Helicobacter pylori cag pathogenicity island’s role in B7-H1 induction and immune evasion. PLoS ONE 2015, 10, e0121841. [Google Scholar] [CrossRef]
- Gobert, A.P.; Verriere, T.; Asim, M.; Barry, D.P.; Piazuelo, M.B.; de Sablet, T.; Delgado, A.G.; Bravo, L.E.; Correa, P.; Peek, R.M., Jr.; et al. Heme oxygenase-1 dysregulates macrophage polarization and the immune response to Helicobacter pylori. J. Immunol. 2014, 193, 3013–3022. [Google Scholar] [CrossRef]
- Kos, F.J.; Engleman, E.G. Immune regulation: A critical link between NK cells and CTLS. Immunol. Today 1996, 17, 174–176. [Google Scholar] [CrossRef]
- Hazenberg, M.D.; Spits, H. Human innate lymphoid cells. Blood 2014, 124, 700–709. [Google Scholar] [CrossRef]
- Guidoboni, M.; Doglioni, C.; Laurino, L.; Boiocchi, M.; Dolcetti, R. Activation of infiltrating cytotoxic T lymphocytes and lymphoma cell apoptotic rates in gastric MALT lymphomas: Differences between high-grade and low-grade cases. Am. J. Pathol. 1999, 155, 823–829. [Google Scholar] [CrossRef]
- Kuo, S.H.; Chen, L.T.; Chen, C.L.; Doong, S.L.; Yeh, K.H.; Wu, M.S.; Mao, T.L.; Hsu, H.C.; Wang, H.P.; Lin, J.T.; et al. Expression of CD86 and increased infiltration of NK cells are associated with Helicobacter pylori-dependent state of early stage high-grade gastric MALT lymphoma. World J. Gastroenterol. 2005, 11, 4357–4362. [Google Scholar] [CrossRef]
- Sonnenberg, G.F. Regulation of intestinal health and disease by innate lymphoid cells. Int. Immunol. 2014, 26, 501–507. [Google Scholar] [CrossRef]
- Vivier, E.; Artis, D.; Colonna, M.; Diefenbach, A.; Di Santo, J.P.; Eberl, G.; Koyasu, S.; Locksley, R.M.; McKenzie, A.N.J.; Mebius, R.E.; et al. Innate Lymphoid Cells: 10 Years On. Cell 2018, 174, 1054–1066. [Google Scholar] [CrossRef]
- Mebius, R.E. Organogenesis of lymphoid tissues. Nat. Rev. Immunol. 2003, 3, 292–303. [Google Scholar] [CrossRef]
- Pearson, C.; Uhlig, H.H.; Powrie, F. Lymphoid microenvironments and innate lymphoid cells in the gut. Trends Immunol. 2012, 33, 289–296. [Google Scholar] [CrossRef]
- Yang, L.; Yamamoto, K.; Nishiumi, S.; Nakamura, M.; Matsui, H.; Takahashi, S.; Dohi, T.; Okada, T.; Kakimoto, K.; Hoshi, N.; et al. Interferon-γ-producing B cells induce the formation of gastric lymphoid follicles after Helicobacter suis infection. Mucosal Immunol. 2015, 8, 279–295. [Google Scholar] [CrossRef]
- Chonwerawong, M.; Avé, P.; Huerre, M.; Ferrero, R.L. Interferon-γ promotes gastric lymphoid follicle formation but not gastritis in Helicobacter-infected BALB/c mice. Gut Pathog. 2016, 8, 61. [Google Scholar] [CrossRef]
- Ikeda, H.; Old, L.J.; Schreiber, R.D. The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Fact. Rev. 2002, 13, 95–109. [Google Scholar] [CrossRef]
- Tian, Z.; van Velkinburgh, J.C.; Wu, Y.; Ni, B. Innate lymphoid cells involve in tumorigenesis. Int. J. Cancer 2016, 138, 22–29. [Google Scholar] [CrossRef]
- Le Bourhis, L.; Guerri, L.; Dusseaux, M.; Martin, E.; Soudais, C.; Lantz, O. Mucosal-associated invariant T cells: Unconventional development and function. Trends Immunol. 2011, 32, 212–218. [Google Scholar] [CrossRef]
- D’Souza, C.; Pediongco, T.; Wang, H.; Scheerlinck, J.Y.; Kostenko, L.; Esterbauer, R.; Stent, A.W.; Eckle, S.B.G.; Meehan, B.S.; Strugnell, R.A.; et al. Mucosal-associated invariant t cells augment immunopathology and gastritis in chronic Helicobacter pylori infection. J. Immunol. 2018, 200, 1901–1916. [Google Scholar]
- Booth, J.S.; Salerno-Goncalves, R.; Blanchard, T.G.; Patil, S.A.; Kader, H.A.; Safta, A.M.; Morningstar, L.M.; Czinn, S.J.; Greenwald, B.D.; Sztein, M.B. Mucosal-Associated Invariant T Cells in the Human Gastric Mucosa and Blood: Role in Helicobacter pylori Infection. Front. Immunol. 2015, 6, 466. [Google Scholar] [CrossRef]
- Burjanadze, M.; Matthes, T.; McKee, T.; Passweg, J.; Huard, B. In situ detection of APRIL-rich niches for plasma-cell survival and their contribution to B-cell lymphoma development. Histol. Histopathol. 2009, 24, 1061–1066. [Google Scholar]
- Kimberley, F.C.; Medema, J.P.; Hahne, M. APRIL in B-cell malignancies and autoimmunity. Results Probl. Cell Differ. 2009, 49, 161–182. [Google Scholar]
- Munari, F.; Lonardi, S.; Cassatella, M.A.; Doglioni, C.; Cangi, M.G.; Amedei, A.; Facchetti, F.; Eishi, Y.; Rugge, M.; Fassan, M.; et al. Tumor-associated macrophages as major source of APRIL in gastric MALT lymphoma. Blood 2011, 117, 6612–6616. [Google Scholar] [CrossRef]
- Floch, P.; Izotte, J.; Guillemaud, J.; Sifré, E.; Costet, P.; Rousseau, B.; Laur, A.M.; Giese, A.; Korolik, V.; Mégraud, F.; et al. A New Animal Model of Gastric Lymphomagenesis: APRIL Transgenic Mice Infected by Helicobacter Species. Am. J. Pathol. 2017, 187, 1473–1484. [Google Scholar] [CrossRef]
- Yokoyama, K.; Higashi, H.; Ishikawa, S.; Fujii, Y.; Kondo, S.; Kato, H.; Azuma, T.; Wada, A.; Hirayama, T.; Aburatani, H.; et al. Functional antagonism between Helicobacter pylori CagA and vacuolating toxin VacA in control of the NFAT signaling pathway in gastric epithelial cells. Proc. Natl. Acad. Sci. USA 2005, 102, 9661–9666. [Google Scholar] [CrossRef]
- Medyouf, H.; Ghysdael, J. The calcineurin/NFAT signaling pathway: A novel therapeutic target in leukemia and solid tumors. Cell Cycle 2008, 7, 297–303. [Google Scholar] [CrossRef]
- Gachet, S.; Ghysdael, J. Calcineurin/NFAT signaling in lymphoid malignancies. Gen. Physiol. Biophys. 2009, 28, F47–F54. [Google Scholar]
- Marafioti, T.; Pozzobon, M.; Hansmann, M.L.; Ventura, R.; Pileri, S.A.; Roberton, H.; Gesk, S.; Gaulard, P.; Barth, T.F.; Du, M.Q.; et al. The NFATc1 transcription factor is widely expressed in white cells and translocates from the cytoplasm to the nucleus in a subset of human lymphomas. Br. J. Haematol 2005, 128, 333–342. [Google Scholar] [CrossRef]
- Akimzhanov, A.; Krenacs, L.; Schlegel, T.; Klein-Hessling, S.; Bagdi, E.; Stelkovics, E.; Kondo, E.; Chuvpilo, S.; Wilke, P.; Avots, A.; et al. Epigenetic changes and suppression of the nuclear factor of activated T cell 1 (NFATC1) promoter in human lymphomas with defects in immunoreceptor signaling. Am. J. Pathol. 2008, 172, 215–224. [Google Scholar] [CrossRef]
- Kondo, E.; Harashima, A.; Takabatake, T.; Takahashi, H.; Matsuo, Y.; Yoshino, T.; Orita, K.; Akagi, T. NF-ATc2 induces apoptosis in Burkitt’s lymphoma cells through signaling via the B cell antigen receptor. Eur. J. Immunol. 2003, 33, 1–11. [Google Scholar] [CrossRef]
- Kuo, S.H.; Tasi, H.J.; Yeh, K.H.; Lin, C.W.; Zeng, Y.S.; Wu, M.S.; Hsu, P.N.; Chen, L.T.; Cheng, A.L. CagA and NFAT co-operatively participate in the lymphomagenesis of gastric MALT lymphoma. Ann. Oncol. 2016, 27 (Suppl. 6), vi313–vi327. [Google Scholar] [CrossRef]
- Wang, H.P.; Zhu, Y.L.; Shao, W. Role of Helicobacter pylori virulence factor cytotoxin-associated gene A in gastric mucosa-associated lymphoid tissue lymphoma. World J. Gastroenterol. 2013, 19, 8219–8226. [Google Scholar] [CrossRef]
- Floch, P.; Mégraud, F.; Lehours, P. Helicobacter pylori Strains and Gastric MALT Lymphoma. Toxins 2017, 9, 132. [Google Scholar] [CrossRef]
- Du, M.Q. MALT lymphoma: A paradigm of NF-κB dysregulation. Semin. Cancer Biol. 2016, 39, 49–60. [Google Scholar] [CrossRef]
- Morgner, A.; Lehn, N.; Andersen, L.P.; Thiede, C.; Bennedsen, M.; Trebesius, K.; Neubauer, B.; Neubauer, A.; Stolte, M.; Bayerdörffer, E. Helicobacter heilmannii-associated primary gastric low-grade MALT lymphoma: Complete remission after curing the infection. Gastroenterology 2000, 118, 821–828. [Google Scholar] [CrossRef]
- Okiyama, Y.; Matsuzawa, K.; Hidaka, E.; Sano, K.; Akamatsu, T.; Ota, H. Helicobacter heilmannii infection: Clinical, endoscopic and histopathological features in Japanese patients. Pathol. Int. 2005, 55, 398–404. [Google Scholar] [CrossRef]
- Okamura, T.; Iwaya, Y.; Yokosawa, S.; Suga, T.; Arakura, N.; Matsumoto, T.; Ogiwara, N.; Higuchi, K.; Ota, H.; Tanaka, E. A case of Helicobacter heilmannii-associated primary gastric mucosa-associated lymphoid tissue lymphoma achieving complete remission after eradication. Clin. J. Gastroenterol. 2013, 6, 38–45. [Google Scholar] [CrossRef]
- O’Rourke, J.L.; Dixon, M.F.; Jack, A.; Enno, A.; Lee, A. Gastric B-cell mucosa-associated lymphoid tissue (MALT) lymphoma in an animal model of ‘Helicobacter heilmannii’ infection. J. Pathol. 2004, 203, 896–903. [Google Scholar] [CrossRef]
- Enno, A.; O’Rourke, J.L.; Howlett, C.R.; Jack, A.; Dixon, M.F.; Lee, A. MALToma-like lesions in the murine gastric mucosa after long-term infection with Helicobacter felis. A mouse model of Helicobacter pylori-induced gastric lymphoma. Am. J. Pathol. 1995, 147, 217–222. [Google Scholar]
- Enno, A.; O’Rourke, J.; Braye, S.; Howlett, R.; Lee, A. Antigen-dependent progression of mucosa-associated lymphoid tissue (MALT)-type lymphoma in the stomach. Effects of antimicrobial therapy on gastric MALT lymphoma in mice. Am. J. Pathol. 1998, 152, 1625–1632. [Google Scholar]
- Zucca, E.; Bertoni, F. The spectrum of MALT lymphoma at different sites: Biological and therapeutic relevance. Blood 2016, 127, 2082–2092. [Google Scholar] [CrossRef]
- Ponzoni, M.; Ferreri, A.J. Bacteria associated with marginal zone lymphomas. Best Pract. Res. Clin. Haematol. 2017, 30, 32–40. [Google Scholar] [CrossRef]
- Kluin, P.M.; Langerak, A.W.; Beverdam-Vincent, J.; Geurts-Giele, W.R.; Visser, L.; Rutgers, B.; Schuuring, E.; Van Baarlen, J.; Lam, K.H.; Seldenrijk, K.; et al. Paediatric nodal marginal zone B-cell lymphadenopathy of the neck: A Haemophilus influenzae-driven immune disorder? J. Pathol. 2015, 236, 302–314. [Google Scholar] [CrossRef]
- Willerslev-Olsen, A.; Krejsgaard, T.; Lindahl, L.M.; Litvinov, I.V.; Fredholm, S.; Petersen, D.L.; Nastasi, C.; Gniadecki, R.; Mongan, N.P.; Sasseville, D.; et al. Staphylococcal enterotoxin A (SEA) stimulates STAT3 activation and IL-17 expression in cutaneous T-cell lymphoma. Blood 2016, 127, 1287–1296. [Google Scholar] [CrossRef]
- Kim, M.; Kim, C.H. Regulation of humoral immunity by gut microbial products. Gut Microbes 2017, 8, 392–399. [Google Scholar] [CrossRef]
- Richard, M.L.; Sokol, H. The gut mycobiota: Insights into analysis, environmental interactions and role in gastrointestinal diseases. Nat. Rev. Gastroenterol. Hepatol. 2019. [Google Scholar] [CrossRef]
- Yamamoto, M.L.; Schiestl, R.H. Lymphoma caused by intestinal microbiota. Int. J. Environ. Res. Public Health 2014, 11, 9038–9049. [Google Scholar] [CrossRef]
- Wei, B.; Su, T.T.; Dalwadi, H.; Stephan, R.P.; Fujiwara, D.; Huang, T.T.; Brewer, S.; Chen, L.; Arditi, M.; Borneman, J.; et al. Resident enteric microbiota and CD8+ T cells shape the abundance of marginal zone B cells. Eur. J. Immunol. 2008, 38, 3411–3425. [Google Scholar] [CrossRef]
- Yamamoto, M.L.; Maier, I.; Dang, A.T.; Berry, D.; Liu, J.; Ruegger, P.M.; Yang, J.I.; Soto, P.A.; Presley, L.L.; Reliene, R.; et al. Intestinal bacteria modify lymphoma incidence and latency by affecting systemic inflammatory state, oxidative stress, and leukocyte genotoxicity. Cancer Res. 2013, 73, 4222–4232. [Google Scholar] [CrossRef]
- Sgouras, D.N.; Panayotopoulou, E.G.; Martinez-Gonzalez, B.; Petraki, K.; Michopoulos, S.; Mentis, A. Lactobacillus johnsonii La1 attenuates Helicobacter pylori-associated gastritis and reduces levels of proinflammatory chemokines in C57BL/6 mice. Clin. Diagn. Lab. Immunol. 2005, 12, 1378–1386. [Google Scholar] [CrossRef]
- Isobe, H.; Nishiyama, A.; Takano, T.; Higuchi, W.; Nakagawa, S.; Taneike, I.; Fukushima, Y.; Yamamoto, T. Reduction of overall Helicobacter pylori colonization levels in the stomach of Mongolian gerbil by Lactobacillus johnsonii La1 (LC1) and its in vitro activities against H. pylori motility and adherence. Biosci. Biotechnol. Biochem. 2012, 76, 850–852. [Google Scholar] [CrossRef]
- Aiba, Y.; Ishikawa, H.; Tokunaga, M.; Komatsu, Y. Anti-Helicobacter pylori activity of non-living, heat-killed form of lactobacilli including Lactobacillus johnsonii No.1088. FEMS Microbiol. Lett. 2017, 364. [Google Scholar] [CrossRef]


| Indirect and Direct Lymphomagenesis-Related Signaling | Makers | Methods | Reference |
|---|---|---|---|
| Intratumor T cells | CD40/CD40L | IHC | 25,31 |
| Co-stimulatory molecules | CD86 (B7.2) | IHC | 40,100 |
| CD4+CD56+ regulatory T-cell | FOXP3 | IHC | 62,63,64 |
| Chemokine receptor | CCL17 and CCL20 | IHC | 62 |
| Chemokines and receptor | BCA-1/CXCR5 | IHC | 49 |
| Chemokines | IL-22 | IHC | 81 |
| Methylation | p16INK4A | Methylation-Specific PCR | 71,73 |
| Natural killer cell | CD56 | IHC | 100 |
| HP-specific protein | CagA protein | IHC | 17,27 |
| HP-specific protein | Serum CagA IgG antibody | ELISA (a CagA kit) | 89 |
| CagA-signaling molecules | p-SHP-2, p-ERK, Bcl-2 | IHC | 91 |
| CagA-signaling molecules | NFATc1 | IHC | 119,120,122 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, S.-H.; Wu, M.-S.; Yeh, K.-H.; Lin, C.-W.; Hsu, P.-N.; Chen, L.-T.; Cheng, A.-L. Novel Insights of Lymphomagenesis of Helicobacter pylori-Dependent Gastric Mucosa-Associated Lymphoid Tissue Lymphoma. Cancers 2019, 11, 547. https://doi.org/10.3390/cancers11040547
Kuo S-H, Wu M-S, Yeh K-H, Lin C-W, Hsu P-N, Chen L-T, Cheng A-L. Novel Insights of Lymphomagenesis of Helicobacter pylori-Dependent Gastric Mucosa-Associated Lymphoid Tissue Lymphoma. Cancers. 2019; 11(4):547. https://doi.org/10.3390/cancers11040547
Chicago/Turabian StyleKuo, Sung-Hsin, Ming-Shiang Wu, Kun-Huei Yeh, Chung-Wu Lin, Ping-Ning Hsu, Li-Tzong Chen, and Ann-Lii Cheng. 2019. "Novel Insights of Lymphomagenesis of Helicobacter pylori-Dependent Gastric Mucosa-Associated Lymphoid Tissue Lymphoma" Cancers 11, no. 4: 547. https://doi.org/10.3390/cancers11040547