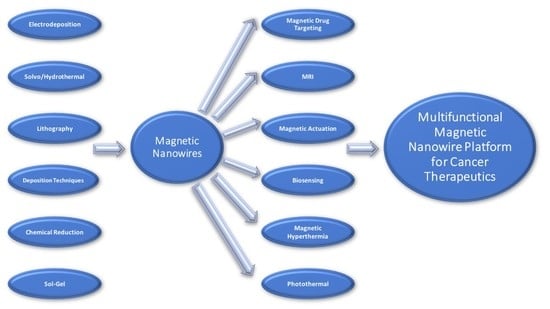
Multifunctional Magnetic Nanowires: Design, Fabrication, and Future Prospects as Cancer Therapeutics
Abstract
:1. Introduction
2. Considerations and Applications of Magnetic Nanowires for Cancer Therapeutics
2.1. Considerations of the Microenvironment of Cancerous Tissue for the Design of Magnetic Nanowire Therapeutic Systems
2.2. Cancer Therapeutic Applications Employing Magnetic Nanowires
2.2.1. The Application of Magnetic Nanowires as Magnetic Drug Targeting Agents in Cancer Therapeutics
2.2.2. The Application of Magnetic Nanowires as Hyperthermic Agents in Cancer Therapeutics
2.2.3. The Application of Magnetic Nanowires as Magnetic Actuation Agents in Cancer Therapeutics
2.3. Magnetic Multifunctional Nanowire Systems in Cancer Therapeutics
2.3.1. The Use of Magnetic Nanowire Magnetic-Chemo-Photothermal Systems in Cancer Therapeutics
2.3.2. The Use of Magnetic Nanowire Magnetic Actuation-Chemotherapeutic Systems in Cancer Therapeutics
2.3.3. The Use of Magnetic Nanowire as a Theranostics System in Cancer
3. NW Fabrication and Synthesis of Magnetic Nanowire Drug Delivery Systems
3.1. Fabrication Methods of Magnetic Nanowires
3.1.1. Electrodeposition of Magnetic Nanowires
3.1.2. Pulsed Laser Deposition of Magnetic Nanowires
3.1.3. Other Synthesis Techniques of Magnetic Nanowires
3.2. Magnetic Properties and Advantages of Nanowires in Drug Delivery Systems
3.3. Stabilization and Functionalization of the Magnetic Nanowires
3.4. Chemotherapeutic Drug Loading and Release of Magnetic Nanowire Systems
4. Cellular Interactions and Toxicity Between Magnetic Nanowires and Cells
4.1. Cellular Internalization of Magnetic Nanowires
4.2. Cellular Toxicity of Magnetic Nanowires and Drug Loaded Magnetic Nanowires
4.3. Cellular Degradation of Magnetic Nanowires
5. Conclusions and Future Prospects
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Y.; Zhao, R.; Wang, S.; Liu, Z.; Tang, R. In vivo Dual-Targeted Chemotherapy of Drug Resistant Cancer by Rationally Designed Nanocarrier. Biomaterials 2016, 75, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Xue, P.; Liu, S.; Zhang, L.; Guan, Q.; Zhu, J.; Tian, X. Metal-Based Hybrid Nanoparticles as Radiosensitizers in Cancer Therapy. Colloid Interface Sci. Commun. 2018, 23, 45–51. [Google Scholar] [CrossRef]
- Rackwitz, T.; Debus, J. Clinical Applications of Proton and Carbon Ion Therapy. Semin. Oncol. 2019, 46, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Peng, F.; Su, Y.; Ji, X.; Zhong, Y.; Wei, X.; He, Y. Doxorubicin-Loaded Silicon Nanowires for the Treatment of Drug-Resistant Cancer Cells. Biomaterials 2014, 35, 5188–5195. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.-Z.; Hoop, M.; Shamsudhin, N.; Huang, T.; Özkale, B.; Li, Q.; Siringil, E.; Mushtaq, F.; Di Tizio, L.; Nelson, B.J.; et al. Hybrid Magnetoelectric Nanowires for Nanorobotic Applications: Fabrication, Magnetoelectric Coupling, and Magnetically Assisted in Vitro Targeted Drug Delivery. Adv. Mater. 2017, 29, 1605458. [Google Scholar] [CrossRef] [PubMed]
- Doucey, M.-A.; Carrara, S. Nanowire Sensors in Cancer. Trends Biotechnol. 2019, 37, 86–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, R.S.; Draheim, R.R.; Roldo, M. Silver Nanowires: Synthesis, Antibacterial Activity and Biomedical Applications. Appl. Sci. 2018, 8, 673. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Wu, A.; Colombi Ciacchi, L.; Wei, G. Recent Advances in Nanoporous Membranes for Water Purification. Nanomaterials 2018, 8, 65. [Google Scholar] [CrossRef] [Green Version]
- Irshad, I.; Ahmad, F.; Mohammed, N. A Review on Nanowires as an Alternative High Density Magnetic Storage Media. AIP Conf. Proc. 2012, 1482, 625–632. [Google Scholar] [CrossRef]
- Bayrak, T.; Jagtap, N.S.; Erbe, A. Review of the Electrical Characterization of Metallic Nanowires on DNA Templates. Int. J. Mol. Sci. 2018, 19, 3019. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Banderas, A.I.; Aires, A.; Teran, F.J.; Perez, J.E.; Cadenas, J.F.; Alsharif, N.; Ravasi, T.; Cortajarena, A.L.; Kosel, J. Functionalized Magnetic Nanowires for Chemical and Magneto-Mechanical Induction of Cancer Cell Death. Sci. Rep. 2016, 6, 35786. Available online: https://www.nature.com/articles/srep35786#supplementary-information (accessed on 12 August 2019). [CrossRef] [PubMed]
- Heidarshenas, B.; Wei, H.; Ali Moghimi, Z.; Hussain, G.; Baniasadi, F.; Naghieh, G. Nanowires in Magnetic Drug Targeting. Mater. Sci. Eng. Int. J. 2019, 3, 3–9. [Google Scholar] [CrossRef]
- Esteban-Fernández de Ávila, B.; Ramírez-Herrera, D.E.; Campuzano, S.; Angsantikul, P.; Zhang, L.; Wang, J. Nanomotor-Enabled Ph-Responsive Intracellular Delivery of Caspase-3: Toward Rapid Cell Apoptosis. ACS Nano 2017, 11, 5367–5374. [Google Scholar] [CrossRef] [PubMed]
- Assunção-Silva, R.C.; Gomes, E.D.; Silva, N.A.; Salgado, A.J. Chapter 8-Nanoengineered Biomaterials for Spinal Cord Regeneration. In Nanoengineered Biomaterials for Regenerative Medicine; Mozafari, M., Rajadas, J., Kaplan, D., Eds.; Elsevier: Boston, MA, USA, 2019; pp. 167–185. [Google Scholar] [CrossRef]
- Pondman, K.; Maijenburg, W.; Celikkol, B.; Pathan, A.A.; Kishore, U.; Ten Haken, B.; Ten Elshof, A. Au Coated Ni Nanowires with Tuneable Dimensions for Biomedical Applications. J. Mater. Chem. 2013, 1, 6129–6136. [Google Scholar] [CrossRef] [Green Version]
- Lisjak, D.; Mertelj, A. Anisotropic Magnetic Nanoparticles: A Review of Their Properties, Syntheses and Potential Applications. Prog. Mater. Sci. 2018, 95, 286–328. [Google Scholar] [CrossRef]
- Pondman, K.M.; Bunt, N.D.; Maijenburg, A.W.; van Wezel, R.J.A.; Kishore, U.; Abelmann, L.; ten Elshof, J.E.; ten Haken, B. Magnetic Drug Delivery with Fepd Nanowires. J. Magn. Magn. Mater. 2015, 380, 299–306. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; Dan, A.; Chakravorty, D. Review Synthesis of Conducting Nanowires. J. Mater. Sci. 2002, 37, 4261–4271. [Google Scholar] [CrossRef]
- Biswas, A.; Bayer, I.S.; Biris, A.S.; Wang, T.; Dervishi, E.; Faupel, F. Advances in Top–Down and Bottom–up Surface Nanofabrication: Techniques, Applications & Future Prospects. Adv. Colloid Interface Sci. 2012, 170, 2–27. [Google Scholar] [CrossRef]
- Mu, Q.; Yan, B. Editorial: Nanoparticles in Cancer Therapy-Novel Concepts, Mechanisms, and Applications. Front. Pharmacol. 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, C.; Suares, D.; Yergeri, M.C. Tumor Microenvironment Targeted Nanotherapy. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef]
- Latorre, A.; Couleaud, P.; Aires, A.; Cortajarena, A.L.; Somoza, Á. Multifunctionalization of Magnetic Nanoparticles for Controlled Drug Release: A General Approach. Eur. J. Med. Chem. 2014, 82, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Hoosen, Y.; Pradeep, P.; Kumar, P.; du Toit, L.C.; Choonara, Y.E.; Pillay, V. Nanotechnology and Glycosaminoglycans: Paving the Way Forward for Ovarian Cancer Intervention. Int. J. Mol. Sci. 2018, 19, 731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, W.; Zheng, Y.; Qian, B.-Z.; Zhao, M. Prostate Cancer Stem Cells and Nanotechnology: A Focus on Wnt Signaling. Front. Pharmacol. 2017, 8, 153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, D.; Ji, X.; Wang, H.; Bin, S.; Chu, B.; Shi, Y.; Su, Y.; He, Y. Silicon Nanowire-Based Multifunctional Platform for Chemo-Photothermal Synergistic Cancer Therapy. J. Mater. Chem. B 2018, 6, 3876–3883. [Google Scholar] [CrossRef]
- Tan, H.; Huang, Y.; Xu, J.; Chen, B.; Zhang, P.; Ye, Z.; Liang, S.; Xiao, L.; Liu, Z. Spider Toxin Peptide Lycosin-I Functionalized Gold Nanoparticles for in Vivo Tumor Targeting and Therapy. Theranostics 2017, 7, 3168–3178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.-W.; Syu, W.-J.; Huang, T.-C.; Lee, Y.-C.; Hsiao, J.-K.; Huang, K.-Y.; Yu, H.-P.; Liao, M.-Y.; Lai, P.-S. Encapsulation of Au/Fe3o4 Nanoparticles into a Polymer Nanoarchitecture with Combined near Infrared-Triggered Chemo-Photothermal Therapy Based on Intracellular Secondary Protein Understanding. J. Mater. Chem. B 2017, 5, 5774–5782. [Google Scholar] [CrossRef]
- Li, T.; Liu, H.; Xi, G.; Pang, Y.; Wu, L.; Wang, X.; Chen, T. One-Step Reduction and Peiylation of Pegylated Nanographene Oxide for Highly Efficient Chemo-Photothermal Therapy. J. Mater. Chem. B 2016, 4, 2972–2983. [Google Scholar] [CrossRef]
- Sensenig, R.; Sapir, Y.; MacDonald, C.; Cohen, S.; Polyak, B. Magnetic Nanoparticle-Based Approaches to Locally Target Therapy and Enhance Tissue Regeneration in Vivo. Nanomedicine 2012, 7, 1425–1442. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.-L.; Chen, D.; Shang, P.; Yin, D.-C. A Review of Magnet Systems for Targeted Drug Delivery. J. Control. Release 2019, 302, 90–104. [Google Scholar] [CrossRef]
- Shen, S.; Wu, Y.; Liu, Y.; Wu, D. High Drug-Loading Nanomedicines: Progress, Current Status, and Prospects. Int. J. Nanomed. 2017, 12, 4085–4109. [Google Scholar] [CrossRef] [Green Version]
- Alsharif, N.; Martinez Banderas, A.; Merzaban, J.; Ravasi, T.; Kosel, J. Biofunctionalizing Magnetic Nanowires toward Targeting and Killing Leukemia Cancer Cells. IEEE Trans. Magn. 2018, 55, 1–5. [Google Scholar] [CrossRef]
- Zhu, H.; Deng, J.; Yang, Y.; Li, Y.; Shi, J.; Zhao, J.; Deng, Y.; Chen, X.; Yang, W. Cobalt Nanowire-Based Multifunctional Platform for Targeted Chemo-Photothermal Synergistic Cancer Therapy. Colloids Surf. B 2019, 180, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Wei, X.; Peng, F.; Zhong, Y.; Lu, Y.; Su, S.; Xu, T.; Lee, S.-T.; He, Y. Gold Nanoparticles-Decorated Silicon Nanowires as Highly Efficient near-Infrared Hyperthermia Agents for Cancer Cells Destruction. Nano Lett. 2012, 12, 1845–1850. [Google Scholar] [CrossRef] [PubMed]
- Egolf, P.W.; Shamsudhin, N.; Pané, S.; Vuarnoz, D.; Pokki, J.; Pawlowski, A.-G.; Tsague, P.; Marco, B.; Bovy, W.; Tucev, S.; et al. Hyperthermia with Rotating Magnetic Nanowires Inducing Heat into Tumor by Fluid Friction. J. Appl. Phys. 2016, 120, 064304. [Google Scholar] [CrossRef]
- Lin, W.-S.; Lin, H.-M.; Chen, H.-H.; Hwu, Y.-K.; Chiou, Y.-J. Shape Effects of Iron Nanowires on Hyperthermia Treatment. J. Nanomater. 2013, 2013, 9. [Google Scholar] [CrossRef] [Green Version]
- Fernandez-Roldan, J.A.; Serantes, D.; del Real, R.P.; Vazquez, M.; Chubykalo-Fesenko, O. Micromagnetic Evaluation of the Dissipated Heat in Cylindrical Magnetic Nanowires. Appl. Phys. Lett. 2018, 112, 212402. [Google Scholar] [CrossRef] [Green Version]
- Contreras, M.F.; Zaher, A.; Perez, J.E.; Ravasi, T.; Kosel, J. Magnetic Nanowires and Hyperthermia: How Geometry and Material Affect Heat Production Efficiency. In Proceedings of the 2015 IEEE International Magnetics Conference (INTERMAG), Beijing, China, 11–15 May 2015. [Google Scholar]
- Alonso, J.; Khurshid, H.; Sankar, V.; Nemati, Z.; Phan, M.H.; Garayo, E.; García, J.A.; Srikanth, H. Feco Nanowires with Enhanced Heating Powers and Controllable Dimensions for Magnetic Hyperthermia. J. Appl. Phys. 2015, 117, 17D113. [Google Scholar] [CrossRef]
- Spirou, S.V.; Basini, M.; Lascialfari, A.; Sangregorio, C.; Innocenti, C. Magnetic Hyperthermia and Radiation Therapy: Radiobiological Principles and Current Practice. Nanomaterials 2018, 8, 401. [Google Scholar] [CrossRef] [Green Version]
- Choi, D.S.; Park, J.; Kim, S.; Gracias, D.H.; Cho, M.K.; Kim, Y.K.; Fung, A.; Lee, S.E.; Chen, Y.; Khanal, S.; et al. Hyperthermia with Magnetic Nanowires for Inactivating Living Cells. J. Nanosci. Nanotechnol. 2008, 8, 2323–2327. [Google Scholar] [CrossRef]
- Hopkins, X.; Gill, W.A.; Kringel, R.; Wang, G.; Hass, J.; Acharya, S.; Park, J.; Jeon, I.T.; An, B.H.; Lee, J.S.; et al. Radio Frequency-Mediated Local Thermotherapy for Destruction of Pancreatic Tumors Using Ni–Au Core–Shell Nanowires. Nanotechnology 2016, 28, 03LT01. [Google Scholar] [CrossRef]
- Contreras, M.F.; Sougrat, R.; Zaher, A.; Ravasi, T.; Kosel, J. Non-Chemotoxic Induction of Cancer Cell Death Using Magnetic Nanowires. Int. J. Nanomed. 2015, 10, 2141–2153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fung, A.; Kapadia, V.; Pierstorff, E.; Ho, D.; Chen, Y. Induction of Cell Death by Magnetic Actuation of Nickel Nanowires Internalized by Fibroblasts. J. Phys. Chem. C 2008, 112. [Google Scholar] [CrossRef]
- Zablotskii, V.; Lunov, O.; Novotna, B.; Churpita, O.; Trosan, P.; Holan, V.; Syková, E.; Dejneka, A.; Kubinova, S. Down-Regulation of Adipogenesis of Mesenchymal Stem Cells by Oscillating High-Gradient Magnetic Fields and Mechanical Vibration. Appl. Phys. Lett. 2014, 105, 103702. [Google Scholar] [CrossRef]
- Serrà, A.; Vázquez-Mariño, G.; García-Torres, J.; Bosch, M.; Vallés, E. Magnetic Actuation of Multifunctional Nanorobotic Platforms to Induce Cancer Cell Death. Adv. Biosyst. 2018, 2, 1700220. [Google Scholar] [CrossRef]
- Nielsch, K.; Wehrspohn, R.; Barthel, J.; Gosele, U.; Fischer, S.; Kronmüller, H. Hexagonally Ordered 100 Nm Period Nickel Nanowire Arrays. Appl. Phys. Lett. 2001, 79, 1360–1362. [Google Scholar] [CrossRef] [Green Version]
- Vega, V.; Böhnert, T.; Martens, S.; Waleczek, M.; Moreno, J.; Görlitz, D.; Prida, V.; Nielsch, K. Tuning the Magnetic Anisotropy of Coni Nanowires: Comparison between Single Nanowires and Nanowire Arrays in Hard-Anodic Aluminum Oxide Membranes. Nanotechnology 2012, 23, 465709. [Google Scholar] [CrossRef]
- Ferré, R.; Ounadjela, K.; George, J.M.; Piraux, L.; Dubois, S. Magnetization Processes in Nickel and Cobalt Electrodeposited Nanowires. Phys. Rev. B 1997, 56, 14066–14075. [Google Scholar] [CrossRef]
- Hergt, R.; Dutz, S.; Müller, R.; Zeisberger, M. Magnetic Particle Hyperthermia: Nanoparticle Magnetism and Materials Development for Cancer Therapy. J. Phys. Condens. Matter 2006, 18, S2919. [Google Scholar] [CrossRef]
- Glöckl, G.; Hergt, R.; Zeisberger, M.; Dutz, S.; Nagel, S.; Weitschies, W. The Effect of Field Parameters, Nanoparticle Properties and Immobilization on the Specific Heating Power in Magnetic Particle Hyperthermia. J. Phys. 2006, 18, S2935. [Google Scholar] [CrossRef]
- Kossatz, S.; Grandke, J.; Couleaud, P.; Latorre, A.; Aires, A.; Crosbie-Staunton, K.; Ludwig, R.; Dahring, H.; Ettelt, V.; Lazaro-Carrillo, A.; et al. Efficient Treatment of Breast Cancer Xenografts with Multifunctionalized Iron Oxide Nanoparticles Combining Magnetic Hyperthermia and Anti-Cancer Drug Delivery. Breast Cancer Res. 2015, 17, 66. [Google Scholar] [CrossRef] [Green Version]
- Gao, W.; Kagan, D.; Pak, O.S.; Clawson, C.; Campuzano, S.; Chuluun-Erdene, E.; Shipton, E.; Fullerton, E.E.; Zhang, L.; Lauga, E.; et al. Cargo-Towing Fuel-Free Magnetic Nanoswimmers for Targeted Drug Delivery. Small 2012, 8, 460–467. [Google Scholar] [CrossRef] [Green Version]
- Gao, W.; de Ávila, B.E.-F.; Zhang, L.; Wang, J. Targeting and Isolation of Cancer Cells Using Micro/Nanomotors. Adv. Drug Deliv. Rev. 2018, 125, 94–101. [Google Scholar] [CrossRef]
- Liu, X.L.; Fan, H.M. Innovative Magnetic Nanoparticle Platform for Magnetic Resonance Imaging and Magnetic Fluid Hyperthermia Applications. Curr. Opin. Chem. Eng. 2014, 4, 38–46. [Google Scholar] [CrossRef]
- Bose, R.J.C.; Lee, S.-H.; Park, H. Biofunctionalized Nanoparticles: An Emerging Drug Delivery Platform for Various Disease Treatments. Drug Discov. Today 2016, 21, 1303–1312. [Google Scholar] [CrossRef]
- Ivanov, Y.P.; Alfadhel, A.; Alnassar, M.; Perez, J.E.; Vazquez, M.; Chuvilin, A.; Kosel, J. Tunable Magnetic Nanowires for Biomedical and Harsh Environment Applications. Sci. Rep. 2016, 6, 24189. [Google Scholar] [CrossRef] [Green Version]
- Martinez Banderas, A. A Combined Chemical and Magneto-Mechanical Induction of Cancer Cell Death by the Use of Functionalized Magnetic Iron Nanowires; King Abdullah University of Science and Technology: Thuwal, Saudi Arabia, 2016. [Google Scholar]
- Sneider, A.; VanDyke, D.; Paliwal, S.; Rai, P. Remotely Triggered Nano-Theranostics for Cancer Applications. Nanotheranostics 2017, 1, 1. [Google Scholar] [CrossRef] [Green Version]
- Lee, N.; Hyeon, T. Designed Synthesis of Uniformly Sized Iron Oxide Nanoparticles for Efficient Magnetic Resonance Imaging Contrast Agents. Chem. Soc. Rev. 2012, 41, 2575–2589. [Google Scholar] [CrossRef]
- Shore, D.; Pailloux, S.L.; Zhang, J.; Gage, T.; Flannigan, D.J.; Garwood, M.; Pierre, V.C.; Stadler, B.J.H. Electrodeposited Fe and Fe–Au Nanowires as Mri Contrast Agents. Chem. Commun. 2016, 52, 12634–12637. [Google Scholar] [CrossRef]
- Bañobre-López, M.; Bran, C.; Rodríguez-Abreu, C.; Gallo, J.; Vázquez, M.; Rivas, J. A Colloidally Stable Water Dispersion of Ni Nanowires as an Efficient T2-Mri Contrast Agent. J. Mater. Chem. B 2017, 5, 3338–3347. [Google Scholar] [CrossRef]
- Staňo, M.; Fruchart, O. Chapter 3-Magnetic Nanowires and Nanotubes. In Handbook of Magnetic Materials; Brück, E., Ed.; Elsevier: Boston, MA, USA, 2018; Volume 27, pp. 155–267. [Google Scholar]
- Sumanth Kumar, D.; Jai Kumar, B.; Mahesh, H.M. Chapter 3-Quantum Nanostructures (Qds): An Overview. In Synthesis of Inorganic Nanomaterials; Mohan Bhagyaraj, S., Oluwafemi, O.S., Kalarikkal, N., Thomas, S., Eds.; Woodhead Publishing: Sawston, UK, 2018; pp. 59–88. [Google Scholar] [CrossRef]
- Li, X.; Sun, L.; Wang, H.; Xie, K.; Long, Q.; Lai, X.; Liao, L. Synthesis of Cobalt Nanowires in Aqueous Solution under an External Magnetic Field. Beilstein J. Nanotechnol. 2016, 7, 990–994. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.-J.; Hwang, S.H.; Jeon, S.; Jung, J.-Y.; Lee, J.; Choi, D.-G.; Choi, J.-H.; Park, S.-H.; Jeong, J.-H. Effects of Polymer Surface Energy on Morphology and Properties of Silver Nanowire Fabricated Via Nanoimprint and E-Beam Evaporation. Appl. Surf. Sci. 2017, 420, 429–438. [Google Scholar] [CrossRef]
- Datta, A.; Sangle, A.; Hardingham, N.; Cooper, C.; Kraan, M.; Ritchie, D.; Narayan, V.; Kar-Narayan, S. Structure and Thermoelectric Properties of Bi2−Xsbxte3 Nanowires Grown in Flexible Nanoporous Polycarbonate Templates. Materials 2017, 10, 553. [Google Scholar] [CrossRef] [Green Version]
- DeMeo, D.; Macnaughton, S.; Sonkusale, S.; Vandervelde, T. Electrodeposited Copper Oxide and Zinc Oxide Core-Shell Nanowire Photovoltaic Cells. In Nanowires-Implementations and Applications; Hashim, A., Ed.; IntechOpen: London, UK, 2011. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Li, J.; Wang, J.; Wu, X.; Yu, C.; Xu, T.; Chang, B.; Sun, H.; Arandiyan, H. Orientation Growth and Magnetic Properties of Electrochemical Deposited Nickel Nanowire Arrays. Catalysts 2019, 9, 152. [Google Scholar] [CrossRef] [Green Version]
- Serrà, A.; Vallés, E. Advanced Electrochemical Synthesis of Multicomponent Metallic Nanorods and Nanowires: Fundamentals and Applications. Appl. Mater. Today 2018, 12, 207–234. [Google Scholar] [CrossRef]
- Oviroh, P.O.; Akbarzadeh, R.; Pan, D.; Coetzee, R.A.M.; Jen, T.-C. New Development of Atomic Layer Deposition: Processes, Methods and Applications. Sci. Technol. Adv. Mater. 2019, 20, 465–496. [Google Scholar] [CrossRef] [Green Version]
- Martín-Palma, R.J.; Lakhtakia, A. Chapter 15-Vapor-Deposition Techniques. In Engineered Biomimicry; Lakhtakia, A., Martín-Palma, R.J., Eds.; Elsevier: Boston, MA, USA, 2013; pp. 383–398. [Google Scholar] [CrossRef]
- Parasuraman, K.; Raghunathan, V. Status of Pulsed Laser Deposition: Challenges and Opportunities. Surf. Eng. 2006, 22, 81–83. [Google Scholar] [CrossRef]
- De Teresa, J.; Fernández-Pacheco, A.; Córdoba, R.; Serrano-Ramón, L.; Sangiao, S.; Ibarra, M. Review of Magnetic Nanostructures Grown by Focused Electron Beam Induced Deposition (Febid). J. Phys. D 2016, 49, 243003. [Google Scholar] [CrossRef]
- Nguyen, D.M.; Bich, H.N.; Hai Anh, P.D.; Ai-Le, P.H.; Bui, Q.B. Vertical Copper Oxide Nanowire Arrays Attached Three-Dimensional Macroporous Framework as a Self-Supported Sensor for Sensitive Hydrogen Peroxide Detection. Arab. J. Chem. 2019. [Google Scholar] [CrossRef]
- Pirouzfar, A.; Seyyed Ebrahimi, S.A. Optimization of Sol–Gel Synthesis of Cofe2o4 Nanowires Using Template Assisted Vacuum Suction Method. J. Magn. Magn. Mater. 2014, 370, 1–5. [Google Scholar] [CrossRef]
- Hamdana, G.; Südkamp, T.; Descoins, M.; Mangelinck, D.; Caccamo, L.; Bertke, M.; Wasisto, H.S.; Bracht, H.; Peiner, E. Towards Fabrication of 3d Isotopically Modulated Vertical Silicon Nanowires in Selective Areas by Nanosphere Lithography. Microelectron. Eng. 2017, 179, 74–82. [Google Scholar] [CrossRef]
- Ferreira, J.M.; Souza, K.P.; Queiroz, F.M.; Costa, I.; Tomachuk, C.R. Electrochemical and Chemical Characterization of Electrodeposited Zinc Surface Exposed to New Surface Treatments. Surf. Coat. Technol. 2016, 294, 36–46. [Google Scholar] [CrossRef]
- Loto, C.A. Electroless Nickel Plating–A Review. Silicon 2016, 8, 177–186. [Google Scholar] [CrossRef]
- Asadian, E.; Ghalkhani, M.; Shahrokhian, S. Electrochemical Sensing Based on Carbon Nanoparticles: A Review. Sens. Actuators B 2019, 293, 183–209. [Google Scholar] [CrossRef]
- Jamkhande, P.G.; Ghule, N.W.; Bamer, A.H.; Kalaskar, M.G. Metal Nanoparticles Synthesis: An Overview on Methods of Preparation, Advantages and Disadvantages, and Applications. J. Drug Deliv. Sci. Technol. 2019, 53, 101174. [Google Scholar] [CrossRef]
- Ghosh, S. Electroless Copper Deposition: A Critical Review. Thin Solid Films 2019, 669, 641–658. [Google Scholar] [CrossRef]
- Possin, G.E. A Method for Forming Very Small Diameter Wires. Rev. Sci. Instrum. 1970, 41, 772–774. [Google Scholar] [CrossRef]
- Pérez-Page, M.; Yu, E.; Li, J.; Rahman, M.; Dryden, D.M.; Vidu, R.; Stroeve, P. Template-Based Syntheses for Shape Controlled Nanostructures. Adv. Colloid Interface Sci. 2016, 234, 51–79. [Google Scholar] [CrossRef] [Green Version]
- Gontad, F.; Caricato, A.P.; Cesaria, M.; Resta, V.; Taurino, A.; Colombelli, A.; Leo, C.; Klini, A.; Manousaki, A.; Convertino, A.; et al. Decoration of Silica Nanowires with Gold Nanoparticles through Ultra-Short Pulsed Laser Deposition. Appl. Surf. Sci. 2017, 418, 430–436. [Google Scholar] [CrossRef]
- Li, H.; Guan, L.; Xu, Z.; Zhao, Y.; Sun, J.; Wu, J.; Xu, N. Synthesis and Characterization of Amorphous Sio2 Nanowires Via Pulsed Laser Deposition Accompanied by N2 Annealing. Appl. Surf. Sci. 2016, 389, 705–712. [Google Scholar] [CrossRef]
- Nikov, R.G.; Dikovska, A.O.; Atanasova, G.B.; Avdeev, G.V.; Nedyalkov, N.N. Magnetic-Field-Assisted Formation of Oriented Nanowires Produced by Pld in Open Air. Appl. Surf. Sci. 2018, 458, 273–280. [Google Scholar] [CrossRef]
- Qiu, Z.; Gong, H.; Yang, X.; Zhang, Z.; Han, J.; Cao, B.; Nakamura, D.; Okada, T. Phosphorus Concentration Dependent Microstructure and Optical Property of Zno Nanowires Grown by High-Pressure Pulsed Laser Deposition. J. Phys. Chem. C 2015, 119, 4371–4378. [Google Scholar] [CrossRef]
- Shkurmanov, A.; Sturm, C.; Hochmuth, H.; Grundmann, M. Growth Kinetics of Ultrathin Zno Nanowires Grown by Pulsed Laser Deposition. Procedia Eng. 2016, 168, 1156–1159. [Google Scholar] [CrossRef]
- Nikov, R.G.; Dikovska, A.O.; Avdeev, G.V.; Amoruso, S.; Ausanio, G.; Nedyalkov, N.N. Pld Fabrication of Oriented Nanowires in Magnetic Field. Appl. Surf. Sci. 2019, 471, 368–374. [Google Scholar] [CrossRef]
- Contreras, M.F.; Ravasi, T.; Kosel, J. Targeted Cancer Cell Death Induced by Induced by Biofunctionalized Magnetic Nanowires. In Proceedings of the 2nd Middle East Conference on Biomedical Engineering, Doha, Qatar, 17–20 February 2014. [Google Scholar]
- Sun, D. Effect of Zeta Potential and Particle Size on the Stability of Sio2 Nanospheres as Carrier for Ultrasound Imaging Contrast Agents. Int. J. Electrochem. Sci. 2016, 11, 8520–8529. [Google Scholar] [CrossRef]
- Hussain, M.; Xie, J.; Hou, Z.; Shezad, K.; Xu, J.; Wang, K.; Gao, Y.; Shen, L.; Zhu, J. Regulation of Drug Release by Tuning Surface Textures of Biodegradable Polymer Microparticles. ACS Appl. Mater. Interfaces 2017, 9, 14391–14400. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, H.; Wu, M.; Yufang, Z.; Wang, D.; Jiao, Z. A Soft–Hard Template Approach Towards Hollow Mesoporous Silica Nanoparticles with Rough Surfaces for Controlled Drug Delivery and Protein Adsorption. J. Mater. Chem. B 2015, 3. [Google Scholar] [CrossRef]
- Peng, F.; Su, Y.; Wei, X.; Lu, Y.; Zhou, Y.; Zhong, Y.; Lee, S.-T.; He, Y. Silicon-Nanowire-Based Nanocarriers with Ultrahigh Drug-Loading Capacity for in Vitro and in Vivo Cancer Therapy. Angew. Chem. Int. Ed. Commun. 2013, 52, 1457–1461. [Google Scholar] [CrossRef]
- Wang, J.; Kumeria, T.; Bezem, M.T.; Wang, J.; Sailor, M.J. Self-Reporting Photoluminescent Porous Silicon Microparticles for Drug Delivery. ACS Appl. Mater. Interfaces 2018, 10, 3200–3209. [Google Scholar] [CrossRef]
- Lorenz, M.R.; Holzapfel, V.; Musyanovych, A.; Nothelfer, K.; Walther, P.; Frank, H.; Landfester, K.; Schrezenmeier, H.; Mailänder, V. Uptake of Functionalized, Fluorescent-Labeled Polymeric Particles in Different Cell Lines and Stem Cells. Biomaterials 2006, 27, 2820–2828. [Google Scholar] [CrossRef]
- Murugan, K.; Choonara, Y.E.; Kumar, P.; Bijukumar, D.; du Toit, L.C.; Pillay, V. Parameters and Characteristics Governing Cellular Internalization and Trans-Barrier Trafficking of Nanostructures. Int. J. Nanomed. 2015, 10, 2191–2206. [Google Scholar] [CrossRef]
- Chithrani, B.D.; Ghazani, A.A.; Chan, W.C. Determining the Size and Shape Dependence of Gold Nanoparticle Uptake into Mammalian Cells. Nano Lett. 2006, 6, 662–668. [Google Scholar] [CrossRef]
- Frohlich, E. The Role of Surface Charge in Cellular Uptake and Cytotoxicity of Medical Nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef] [Green Version]
- Behzadi, S.; Serpooshan, V.; Hamaly, M.; Alkawareek, M.; Dreaden, E.; Brown, D.; Alkilany, A.; Farokhzad, O.; Mahmoudi, M. Cellular Uptake of Nanoparticles: Journey inside the Cell. Chem. Soc. Rev. 2017, 46. [Google Scholar] [CrossRef]
- Panzarini, E.; Mariano, S.; Carata, E.; Mura, F.; Rossi, M.; Dini, L. Intracellular transport of silver and gold nanoparticles and biological responses: An update. Int. J. Mol. Sci. 2018, 19, e1305. [Google Scholar] [CrossRef] [Green Version]
- Awaad, A.; Nakamura, M.; Ishimura, K. Imaging of Size-Dependent Uptake and Identification of Novel Pathways in Mouse Peyer’s Patches Using Fluorescent Organosilica Particles. Nanomedicine 2012, 8, 627–636. [Google Scholar] [CrossRef]
- Prina-Mello, A.; Diao, Z.; Coey, J.M.D. Internalization of Ferromagnetic Nanowires by Different Living Cells. J. Nanobiotechnol. 2006, 4, 9. [Google Scholar] [CrossRef] [Green Version]
- Song, M.M.; Song, W.J.; Bi, H.; Wang, J.; Wu, W.L.; Sun, J.; Yu, M. Cytotoxicity and Cellular Uptake of Iron Nanowires. Biomaterials 2010, 31, 1509–1517. [Google Scholar] [CrossRef]
- Choi, D.; Fung, A.; Moon, H.; Ho, D.; Chen, Y.; Kan, E.; Rheem, Y.; Yoo, B.; Myung, N. Transport of Living Cells with Magnetically Assembled Nanowires. Biomed. Microdevices 2007, 9, 143–148. [Google Scholar] [CrossRef]
- Poland, C.A.; Byrne, F.; Cho, W.S.; Prina-Mello, A.; Murphy, F.A.; Davies, G.L.; Coey, J.M.; Gounko, Y.; Duffin, R.; Volkov, Y.; et al. Length-Dependent Pathogenic Effects of Nickel Nanowires in the Lungs and the Peritoneal Cavity. Nanotoxicology 2012, 6, 899–911. [Google Scholar] [CrossRef]
- Wong, P.K. Mutagenicity of Heavy Metals. Bull. Environ. Contam. Toxicol. 1988, 40, 597–603. [Google Scholar] [CrossRef]
- Žužek Rožman, K.; Pečko, D.; Šturm, S.; Maver, U.; Nadrah, P.; Bele, M.; Kobe, S. Electrochemical Synthesis and Characterization of Fe70pd30 Nanotubes for Drug-Delivery Applications. Mater. Chem. Phys. 2012, 133, 218–224. [Google Scholar] [CrossRef]
- Donaldson, K.; Tran, C.L. An Introduction to the Short-Term Toxicology of Respirable Industrial Fibres. Mutat. Res. Fundam. Mol. Mech. Mutagenesis 2004, 553, 5–9. [Google Scholar] [CrossRef]
- Magrez, A.; Kasas, S.; Salicio, V.; Pasquier, N.; Seo, J.W.; Celio, M.; Catsicas, S.; Schwaller, B.; Forro, L. Cellular Toxicity of Carbon-Based Nanomaterials. Nano Lett. 2006, 6, 1121–1125. [Google Scholar] [CrossRef]
- Rahong, S.; Yasui, T.; Kaji, N.; Baba, Y. Recent Developments in Nanowires for Bio-Applications from Molecular to Cellular Levels. Lab Chip 2016, 16, 1126–1138. [Google Scholar] [CrossRef] [Green Version]
- Jahangirian, H.; Kalantari, K.; Izadiyan, Z.; Rafiee-Moghaddam, R.; Shameli, K.; Webster, T.J. A Review of Small Molecules and Drug Delivery Applications Using Gold and Iron Nanoparticles. Int. J. Nanomed. 2019, 14, 1633–1657. [Google Scholar] [CrossRef] [Green Version]
- Safi, M.; Yan, M.; Guedeau-Boudeville, M.-A.; Conjeaud, H.; Garnier-Thibaud, V.; Boggetto, N.; Baeza-Squiban, A.; Niedergang, F.; Averbeck, D.; Berret, J.-F. Interactions between Magnetic Nanowires and Living Cells: Uptake, Toxicity, and Degradation. ACS Nano 2011, 5, 5354–5364. [Google Scholar] [CrossRef] [Green Version]
- Perez, J.E.; Contreras, M.F.; Vilanova, E.; Felix, L.P.; Margineanu, M.B.; Luongo, G.; Porter, A.E.; Dunlop, I.E.; Ravasi, T.; Kosel, J. Cytotoxicity and Intracellular Dissolution of Nickel Nanowires. Nanotoxicology 2016, 10, 871–880. [Google Scholar] [CrossRef]
- Liu, J.; Yu, M.; Zhou, C.; Zheng, J. Renal Clearable Inorganic Nanoparticles: A New Frontier of Bionanotechnology. Mater. Today 2013, 16, 477–486. [Google Scholar] [CrossRef]





| Fabrication Technique | Top-Down/Bottom-Up | Magnetic NW Composition Achievable by This Technique | Remarks on Technique | References |
|---|---|---|---|---|
| electrodeposition | bottom-up | Fe and Fe-based compounds Co and Co-based compounds Ni and Ni-based compounds | Cost effective enables synthesis of accurate dimensions and complex structure Individualistic growth on non-planar surfaces | [68,69,70] |
| atomic Layer deposition | bottom-up | Fe and Fe oxides Co and Co oxides Ni and Ni oxides | Cost effective enables complex structure synthesis Slow deposition rate | [70,71] |
| chemical vapor deposition | bottom-up | single-crystalline Ni and Ni alloys single-crystalline Co and Co alloys single-crystalline Fe and Fe alloys | Cost effective enables complex structure synthesis Difficult to deposit multicomponent constituents | [70,72] |
| pulsed laser deposition | bottom-up | Fe and Fe oxides Co and Co oxides Ni and Ni oxides | Cost effective enables complex structure synthesis Difficult to control dimensions of NW | [70,73] |
| Focused Electron Beam Induced Deposition | bottom-up | Fe deposits Co deposits | Cost effective enables complex structure synthesis Lack of control in final composition of NW | [70,74] |
| Chemical reduction | bottom-up | Fe and Fe-based materials Ni and Ni-based compounds | Cost effective Difficult to control NW dimensions and morphology | [70,75] |
| Solvothermal | bottom-up | Co and Co-based compounds Ni and Ni-based compounds Fe and Fe-based compounds | Difficult to control NW dimensions and morphology Requires high temperatures and pressures | [70,75] |
| Hydrothermal | bottom-up | Fe and Fe-based compounds Co and Co-based compounds Ni and Ni-based compounds | One step synthesis Difficult to control NW dimensions and morphology | [70,75] |
| Sol-Gel | bottom-up | Fe and Fe-based compounds Co and Co-based compounds Ni and Ni-based compounds | Cost effective scalable Can form defects in products | [70,76] |
| Lithography Techniques | top-down | Fe and Fe-based compounds Co and Co-based compounds Ni and Ni-based compounds | Flexible in designing nanoparticles low resolution high cost | [70,77] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nana, A.B.A.; Marimuthu, T.; Kondiah, P.P.D.; Choonara, Y.E.; Du Toit, L.C.; Pillay, V. Multifunctional Magnetic Nanowires: Design, Fabrication, and Future Prospects as Cancer Therapeutics. Cancers 2019, 11, 1956. https://doi.org/10.3390/cancers11121956
Nana ABA, Marimuthu T, Kondiah PPD, Choonara YE, Du Toit LC, Pillay V. Multifunctional Magnetic Nanowires: Design, Fabrication, and Future Prospects as Cancer Therapeutics. Cancers. 2019; 11(12):1956. https://doi.org/10.3390/cancers11121956
Chicago/Turabian StyleNana, Abu Bakr A., Thashree Marimuthu, Pierre P. D. Kondiah, Yahya E. Choonara, Lisa C. Du Toit, and Viness Pillay. 2019. "Multifunctional Magnetic Nanowires: Design, Fabrication, and Future Prospects as Cancer Therapeutics" Cancers 11, no. 12: 1956. https://doi.org/10.3390/cancers11121956